Leptin acts on target cells after binding to the specific receptors. The first leptin receptor (OB-R) has been cloned in the mouse more than 10 years ago (15) and until now at least 6 isoforms have been identified as multiple alternative splice variants with distinct signaling functions (16). The OB-R as a single-pass membrane receptor exhibits a structural similarity with the class I cytokine receptor family, which includes receptors for interleukin 6, leukemia inhibitory factor or granulocyte colony-stimulating factor. Generally three major classes of OB-R can be distinguished: long (OB-Rb), short (OB-Ra, -Rc, -Rd, -Rf) and soluble (OB-Re). All isoforms characterize identical extracellular domain consisting of 816 amino acids (aa). The short 34 aa transcellular domain is typical for a long and short isoforms, but is absent in soluble OB-Re. The different lengths of intracellular part, approximately 300 aa for a long and more than 30 aa for short isoforms, generally distinguish leptin receptor forms. Several reports indicate that OB-Rb, abundantly expressed in the central nervous system (CNS), participates in intracellular full signal transduction by activation of janus kinase/signal transducer and activator of transcription (JAK/STAT) proteins, whereas short form is unable to stimulate this pathway (17, 18). Initially, OB-Ra was considered as a predominant transporter of leptin by the blood-brain barrier to the CNS (19, 20) but recently several reports indicate important role of the receptor in cellular signal transmission via mitogen-activated protein kinase (MAPK) activation (13, 21).
Leptin receptors have been identified in various peripheral tissues and secretory glands as well as in several regions of the CNS of many species (22-26) and their expression fluctuated depending on physiological status. Our previous results showed the presence of leptin and long form of leptin receptor mRNA in hypothalamus, pituitary (7, 27, 28) and tissues of reproductive tract (unpublished observations) during luteal phase and early pregnancy in pigs. However, there is a lack of data concerning the location of short form of leptin receptor (OB-Rs) mRNA. Therefore, the present study was conducted to determine mRNA expression of OB-Rs in distinct tissues of the central nervous system, pituitary and reproductive tract in the pig during mid- and late-luteal phases of the cycle and early pregnancy (early and late stages of implantation).
Animals. The studies were carried out in accordance with principles and procedures of the Animal Ethics Committee at the University of Warmia and Mazury in Olsztyn, Poland. In the present experiment gilts at 9-10 months of age and 90-110 kg of body weight were divided into four groups (n=3-4 per group) as follows: days 10-12 and 14-16 of the estrous cycle (mid-and late-luteal phases) and 14-16 and 30-32 of gestation (the onset and the end of implantation process).
Immediately after slaughter the hypothalamus has been dissected into medial basal hypothalamus (MBH), preoptic area (POA) and stalk median eminence (SME) as was previously described (29). The pituitary was divided into anterior and posterior lobes. From the reproductive tissues corpus luteum, ovarian stroma, endometrium, myometrium, and trophoblast (from pregnant gilts) have been taken. Tissue samples were immediately frozen in liquid nitrogen and than stored at -70°C until RNA extraction.
RNA extraction and real time RT-PCR. Total RNA from each tissue sample was isolated using RNeasy Mini Kit (Qiagen, Maryland, USA) according to the manufacture's instructions. RNA samples were quantified spectrophotometrically (Lambda Bio 10, Perkin Elmer, USA) and the integrity was confirmed using 1.5 % agarose gel.
The partial porcine sequence of the short form receptor was determined based on human, rat and mouse sequences available in GenBank. Several pairs of primers have been designed to obtain the product containing 222 base pairs (bp) consisting of 169 bp of known porcine region of the long form (Accession No AF092422) and 53 bp of the OB-Rs established by comparison with OB-Rs/OB-Ra sequences of other species (for human U50748, for rat D84126 and AF304191, for mouse U58862). The PCR-product was visualized on agarose gel and sequenced in both directions using ABI PrismTM BigDyeTM Terminator Cycle Sequencing kit (ABI, Prism 3777 DNA sequencer, CA, USA). The sequence of 169 bp fragment of the product exhibited 100% homology with porcine leptin receptor, whereas 53 bp fragment has not been found in GeneBank when compared with porcine genome. However, this fragment indicated 96% homology with human OB-Rs, 86% with rat OB-Ra and 88% with mouse OB-Rs.
Finally, real time RT-PCR was performed using a specific forward
(5'-ACACCGGAATGATGCAGGTCTATATG-3') and reverse
(5'-AGATTGGATTCATCTGCAGTGATCATG-3') primers to amplify the product. Samples were normalized using the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GenBank accession No U48832) with forward (5'-CCTTCATTGACCTCCACTACATGGT-3') and reverse (5'-CCACAACATACGTAGCACCACGATC-3') primers, the size of the product was 183 bp.
Real time RT-PCR for tested genes was performed in a 10-µl final reaction volume using LC FastStart DNA Master SYBR Green I kit (Roche, Switzerland). The amplification reaction was consisting of 3.25 mM of Mn(OAc)2; 0.5 µM of each primer; 3.75 µl of 1xSYBRGreen Master Mix and RNA template. Real time RT-PCR was carried out in duplicates for each sample in the LightCycler 2.0 Instrument with capillary system (Roche, Switzerland) using the following parameters: one cycle of 61°C for 20 min (reverse transcription), then 95°C for 10 min (denaturation), followed by 40-50 cycles (amplification) at 95°C for 10s; 58-59°C for 5s and 72°C for 10s. Melt curve analyses were run with each series to confirm the specificity of the amplified products. To confirm no signal from genomic DNA amplifications without reverse transcriptase (RT) were performed as well. The standard curves were prepared for both tested and housekeeping genes. All expression data were normalized by dividing the amount of target gene by the amount of GAPDH used as control.
Statistical analysis All results are presented in figures as sample (raw) means ± SE. Statistical analyses were performed using Statistica (version 6, StatSoft Inc, Tulsa, OK USA.). Significant differences were established by one-way Anova with least significant differences (LSD) post hoc test and assumed as statistically significant for P
Using quantitative real time RT-PCR the expression of the short form of leptin receptor mRNA has been found in majority of tested tissues including hypothalamus (stalk median eminence), pituitary (anterior and posterior parts) and reproductive tract (corpus luteum, ovarian stroma, endometrium, myometrium). The expression of OB-Rs was undetectable in the medial basal hypothalamus, preoptic area and trophoblast. Furthermore, levels of the transcript fluctuated in distinct tissues depending on the phase (mid- and late-luteal) of the estrous cycle and the day of pregnancy (early and late stages of implantation).
In details, the expression of short form leptin receptor mRNA in SME was quite stable and did not differ between analyzed stages of the cycle and pregnancy (Fig. 1A). In anterior pituitary, OB-Rs mRNA levels were almost identical in mid- and late-luteal phases (8.09±1.50 and 8.54±1.20 arbitrary units, respectively; Fig. 1B). However, significant decrease (P<0.05) was observed during 30-32 days of gestation when compared to earlier stage, day 14-16 of pregnancy (10.98±1.94 vs. 5.39±0.88). In posterior pituitary, the transcript levels were almost doubled higher (P<0.01) during two analyzed periods of pregnancy when compared with two stages of luteal phase (Fig. 1C). There was no differences between mid- and late-luteal stages of the cycle (2.49±0.43 and 2.35±0.36, respectively) and between early and late periods of implantation (4.48±0.84 and 4.39±0.51, respectively).
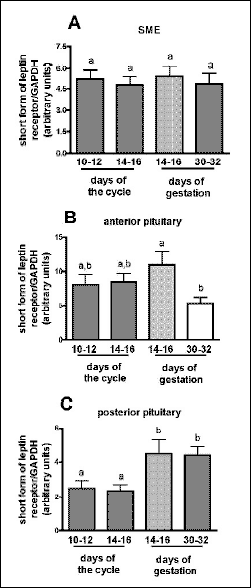 |
Fig. 1. The expression of short form of leptin receptor gene in stalk median eminence (A), anterior (B) and posterior pituitary (C) obtained from gilts during mid- and late-luteal phase of the estrous cycle and early pregnancy (early and late stages of implantation). Each experimental group consisted of 3-4 gilts. The expression of mRNA has been determined by quantitative real time RT-PCR. All expression data were normalized by dividing the amount of target gene by the amount of GAPDH used as control and presented as arbitrary units. Differences in transcript levels were assumed as statistically significant for P<0.05 and marked with different letters. |
Among examined tissues of reproductive tract OB-Rs gene expression has been found in corpus luteum (Fig. 2A). The lowest expression was observed during mid-luteal phase (1.09±0.30 arbitrary units), whereas significantly higher levels were detected in late-luteal stage (4.52±0.34; P<0.05) and gestation (7.71±1.19 and 6.74±0.60 on days14-16 and 30-32, respectively; P<0.01). Additionally, the higher transcripts content was noted during the onset of implantation than during late-luteal phase (Fig. 2A).
In ovarian stroma the expression of mRNA of the leptin receptor was markedly diminished during days 14-16 of the cycle (0.42±0.08) when compared with days: 10-12 of the cycle (1.54±0.24) and 30-32 of gestation (1.15±0.25; P<0.05; Fig. 2B). The tendency to increase (P=0.06) was noted at 14-16 days of pregnancy in relation to 14-16 days of the cycle (1.05±0.27 vs. 0.42±0.08).
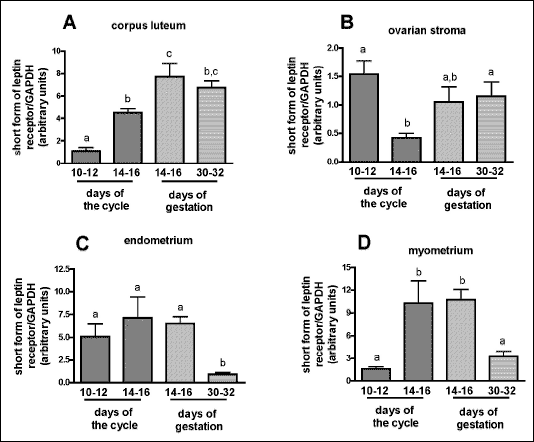 |
| Fig. 2. The expression of short form of leptin receptor gene in corpus luteum (A), ovarian stroma (B), endometrium (C) and myometrium (D) derived from gilts during mid- and late-luteal phase of the estrous cycle and early pregnancy (early and late stages of implantation). Each experimental group consisted of 3-4 gilts per group. The expression of mRNA has been determined by quantitative real time RT-PCR. All expression data were normalized by dividing the amount of target gene by the amount of GAPDH used as control and presented as arbitrary units. Differences in transcript levels were assumed as statistically significant for P<0.05 and marked with different letters. |
The expression of OB-Rs mRNA in endometrium reached the lowest levels on days 30-32 of pregnancy (0,93±0,19 arbitrary units) in comparison with earlier stage (6.52±0.74; P<0.05) and two periods of the cycle (5.09±1.39 and 7.13±2.31, respectively; P<0.05; Fig. 2C). In myometrium the highest levels of the transcript were detected during late-luteal stage of the cycle (10.30±2.94) and 14-16 days of pregnancy (10.75±1.35, Fig. 2D). In remaining periods the transcript levels were significantly diminished to 1.62±0.28 arbitrary units (P<0.05) on days 10-12 of the cycle and 3.25±0.70 (P<0.05) on days 30-32 of gestation.
The study was undertaken to extend our knowledge about the expression of short form leptin receptors in tissues of hypothalamic-pituitary-gonadal axis involved in the regulation of reproductive processes in pigs. Using a very sensitive quantitative real time-PCR method we presented for the first time mRNA of short form of leptin receptor in majority of tested tissues including hypothalamus (stalk median eminence), pituitary (anterior and posterior parts) and reproductive tract (corpus luteum, ovarian stroma, endometrium, myometrium) obtained from gilts during luteal phase of the estrous cycle or early pregnancy. Our study demonstrated that levels of mRNA for OB-Rs varied in distinct tissues depending on the phase (mid- and late-luteal) of the estrous cycle and the day of pregnancy (early and late stages of implantation).
There is no available data describing porcine short form receptors sequences, location and changes in gene expression depend on physiological status of animals. The most attention is concentrated on localization and functions of leptin and/or long form of the receptor, as the one capable to intracellular full signaling transmission. However, short forms should also be considered as very important messengers of leptin action in pigs. Available data indicate that the most common short isoform, OB-Ra, possesses a signaling abilities through mitogen-activated pathway (13, 21, 30), but generally it is not able to activate STAT3 proteins, characteristic for the long form receptor signaling transduction. Additionally, the OB-Ra has been proposed to play a role in leptin transport across the blood-brain barrier (20, 31), where the highest level of the receptor has been detected. As reported in mouse, majority of leptin receptor transcripts, found in nearly all tissues, encoded predominantly short forms, whereas long form was expressed at much lower levels (32). The one exception is hypothalamus, where OB-Rb transcript contents are markedly higher (32). Numerous previous results indicate the presence of OB-Rb mRNA in hypothalamic regions involved in feeding behavior/energy balance and reproduction in pigs (24, 33). Our previously published data showed the high levels of OB-Rb mRNA in all hypothalamic areas involved in GnRH synthesis/release including medial basal hypothalamus, preoptic area, stalk median eminence (7). Interestingly, in the present study we detected OB-Rs mRNA in the SME only, but not in the POA and MBH. Additionally, this hypothalamic region was the only one where transcript contents were constant and did not fluctuate depend on luteal or gestation stages.
Our data showed the presence of mRNA of OB-Rs in anterior pituitary and this observation was supported by others in rodents and cows (26, 34). The OB-Rs mRNA levels were similar in two tested periods of the cycle and at 14-16 days of pregnancy, but markedly decreased during later stage, 30-32 day of gestation. Numerous data underline an important role of leptin and its long form of receptor in the regulation of pituitary hormone secretion in various species, including pigs (4), but there is limited information about functional significance of OB-Rs at various levels of HPG axis in pigs and other species as well. In the present study the receptor mRNA expression was also observed in posterior pituitary. Interestingly, significantly higher levels of OB-Rs were noted during pregnancy, at two stages critical for implantation. At that time it is difficult to speculate possible role of OB-Rs in this gland, because so far there is no evidence describing the influence of leptin on posterior pituitary functions.
This study demonstrated the presence of OB-Rs mRNA expression in corpus luteum, ovarian stroma, endometrium and myometrium of gilts during mid- and late-luteal phases of the cycle and early gestation, but surprisingly, no detectable levels of OB-Rs transcript were found in trophoblast. Our results indicate that levels of OB-Rs mRNA in corpus luteum significantly increased during pregnancy, when compared with luteal phase, but no difference between days 14-16 and 30-32 of gestation was observed. In opposite, in endometrium and miometrium OB-Rs mRNA contents were similarly, significantly decreased at late stage of implantation (30-32 days of pregnancy) when compared with earlier period (14-16 day of gestation) or luteal phase of the cycle, markedly suggesting the role of the receptor during onset of implantation, a critical period for embryos survival. Studies on pigs suggested correlation between ovarian expression of OB-R and progesterone concentration (22). Leptin exerts stimulatory effect on luteal functions, and its mRNA and protein contents enhance, when progesterone accumulation increases in vitro (22, 35, 36). It has been widely reviewed that leptin plays a crucial role during early stages of pregnancy in mammals, affecting fetal and maternal tissues through autocrine and/or paracrine mechanisms (37-40). Whereas leptin and OB-Rb mRNA/proteins were detected in ovary, placenta, uterus and embryos in various species, supporting their role in oocyte maturation, preimplantation/implantation processeses and fetal growth and development (37, 41-44), there are limited data concerning the presence and functions of OB-Rs in above processes. So far, short form of leptin receptor transcripts were detected in various reproductive tissues including preovulatory follicles, corpus luteum, placenta and uterus in rodents, human and baboons (44-47). It has also been reported that their levels fluctuated depend on concentrations of circulating steroid hormones and leptin during the estrous cycle or pregnancy (26, 48, 49). In one study the authors suggested that OB-Ra, found in rat placenta, might participate in transport of leptin into fetus (50). In another report, short form of leptin receptor, probably dominantly expressed in porcine oocyte from medium follicles, was capable to transduce intracellular signal via MAPK pathway after leptin binding (13). In the same study, the AMPK activation enhanced, whereas inhibitor of MAPK blocked porcine nuclear oocyte maturation (13), strongly suggesting possible role of OB-Ra in above process, but additionally in other reproductive function in pigs. Although, the importance of short form leptin receptor in all tested tissues has not been exclusively investigated, the significant role of OB-Ra in the influx of leptin into target cell and involvement in intracellular signal transmission can not be ruled out. However, the functional importance of short form of leptin receptor in pigs remains to be elucidated.
In conclusion, our data demonstrated for the first time the expression of short form of leptin receptor mRNA in discrete areas of porcine hypothalamus, pituitary and reproductive tissues during luteal phase and early pregnancy. The levels of mRNA for this receptor fluctuated in distinct tissues depending on the phase (mid- and late-luteal) of the estrous cycle and the day of pregnancy (early and late stages of implantation), providing evidence for its potential physiological role in leptin influence on reproductive processes in pigs. Furthermore, quantification of receptor mRNA transcripts in various tissues depending on physiological status of gilts provides basement for the future to study the importance of these receptors in pigs.
Acknowledgments: This research was supported by the State Committee for Scientific Research (project No 0206.0805). The authors thank the Roche Company for kindly provided the LightCycler Instrument for real time PCR and Tyminski Karol for helpful advice. We also thank Marta Ślusarz and Marek Łaszyn for technical assistance.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;72:425-432.
- Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol 1999;20:317-363.
- Smith GD, Jackson LM, Foster DL. Leptin regulation of reproductive function and fertility. Theriogenology 2002;57:73-86.
- Barb CR, Hausman GJ, Czaja K. Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol 2005;29:186-192.
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology 1999;140:5995-5998.
- Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 2000;141:333-339.
- Kaminski T, Smolinska N, Gajewska A, Siawrys G, Okrasa S, Kochman K, Przala J. Leptin and long form of leptin receptor genes expression in the hypothalamus and pituitary during the luteal phase and early pregnancy in pigs. J Physiol Pharmacol 2006;57:95-108.
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 1998;47:294-297.
- Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A 1997;94:1023-1028.
- Carro E, Pinilla L, Seoane LM, Considine RV, Aguilar E, Casanueva FF, Dieguez C. Influence of endogenous leptin tone on the estrous cycle and luteinizing hormone pulsatility in female rats. Neuroendocrinology 1997;66:375-377.
- Barb CR, Barrett JB, Kraeling RR. Role of leptin in modulating the hypothalamic-pituitary axis and luteinizing hormone secretion in the prepuberal gilt. Domest Anim Endocrinol 2004;26:201-214.
- Barb CR, Kraeling RR Role of leptin in the regulation of gonadotropin secretion in farm animals. Anim Reprod Sci 2004;82-83:155-167.
- Craig J, Zhu H, Dyce PW, Petrik J, Li J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004;145:5355-5363.
- Zieba DA, Amstalden M, Williams GL. Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest Anim Endocrinol 2005;29:166-185.
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263-1271.
- Tartaglia LA. The leptin receptor. J Biol Chem 1997;272:6093-6096.
- Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 1997;272:32686-695.
- Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm 2005;71:345-372.
- Peiser C, McGregor GP, Lang RE. Binding and internalization of leptin by porcine choroid plexus cells in culture. Neurosci Lett 2000;283:209-212.
- Kastin AJ, Pan W. Dynamic regulation of leptin entry into brain by the blood-brain barrier. Regul Pept 2000;92:37-43.
- Murakami T, Yamashita T, Iida M, Kuwajima M, Shima K. A short form of leptin receptor performs signal transduction. Biochem Biophys Res Commun 1997;231:26-29.
- Ruiz-Cortes ZT, Men T, Palin MF, Downey BR, Lacroix DA, Murphy BD. Porcine leptin receptor: molecular structure and expression in the ovary. Mol Reprod Dev 2000;56:465-474.
- Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB. Long form leptin receptor mRNA expression in the brain, pituitary, and other tissues in the pig. Domest Anim Endocrinol 2000;19:53-61.
- Czaja K, Lakomy M, Sienkiewicz W, Kaleczyc J, Pidsudko Z, Barb CR, Rampacek GB, Kraeling RR. Distribution of neurons containing leptin receptors in the hypothalamus of the pig. Biochem Biophys Res Commun 2002;298:333-337.
- Morash BA, Imran A, Wilkinson D, Ur E, Wilkinson M. Leptin receptors are developmentally regulated in rat pituitary and hypothalamus. Mol Cell Endocrinol 2003;210:1-8.
- Szczepankiewicz D, Wojciechowicz T, Kaczmarek P, Nowak KW. Leptin and its receptors in the course of pregnancy in the rat. Int J Mol Med 2006;17:95-99.
- Siawrys G, Przala J, Kaminski T, Smolinska N, Gajewska A, Kochman K, Skowronski M, Staszkiewicz J. Long form leptin receptor mRNA expression in the hypothalamus and pituitary during early pregnancy in the pig. Neuro Endocrinol Lett 2005;26:305-309.
- Smolinska N, Przala J, Kaminski T, Siawrys G, Gajewska A, Kochman K, Okrasa S. Leptin gene expression in the hypothalamus and pituitary of pregnant pigs. Neuro Endocrinol Lett 2004;25:191-195.
- Sesti LA, Britt JH. Relationship of secretion of GnRH in vitro to changes in pituitary concentrations of LH and FSH and serum concentrations of LH during lactation in sows. J Reprod Fertil 1993;98:393-400.
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int 2004;28:159-169.
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 2002; 143:775-783.
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A 1996;93:6231-6235.
- Lin J, Richard Barb C, Kraeling RR, Rampacek GB. Developmental changes in the long form leptin receptor and related neuropeptide gene expression in the pig brain. Biol Reprod 2001;64:1614-1618.
- Chelikani PK, Glimm DR, Kennelly JJ. Short communication: Tissue distribution of leptin and leptin receptor mRNA in the bovine. J Dairy Sci 2003;86:2369-2372.
- Ruiz-Cortes ZT, Martel-Kennes Y, Gevry NY, Downey BR, Palin MF, Murphy BD. Biphasic effects of leptin in porcine granulosa cells. Biol Reprod 2003;68:789-796.
- Pescador N, Stocco DM, Murphy BD. Growth factor modulation of steroidogenic acute regulatory protein and luteinization in the pig ovary. Biol Reprod 1999;60:1453-1461.
- Ashworth CJ, Hoggard N, Thomas L, Mercer JG, Wallace JM, Lea RG. Placental leptin. Rev Reprod 2000;5:18-24.
- Henson MC, Castracane VD. Leptin in pregnancy. Biol Reprod 2000;63:1219-1228.
- Henson MC, Castracane VD. Placental leptin. Rev Reprod 2000;5:18-24.
- Spicer LJ. Leptin: a possible metabolic signal affecting reproduction. Domest Anim Endocrinol 2001;21:251-270.
- Gonzalez RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, Simon C. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J Clin Endocrinol Metab 2000;85:4883-4888.
- Chen SC, Cunningham JJ, Smeyne RJ. Expression of OB receptor splice variants during prenatal development of the mouse. J Recept Signal Transduct Res 2000; 20:87-103.
- Craig JA, Zhu H, Dyce PW, Wen L, Li J. Leptin enhances porcine preimplantation embryo development in vitro. Mol Cell Endocrinol 2005;229:141-147.
- Yoon SJ, Cha KY, Lee KA. Leptin receptors are down-regulated in uterine implantation sites compared to interimplantation sites. Mol Cell Endocrinol 2005;232:27-35.
- Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod 1997;3:467-472.
- Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab 1999;84:1072-1076.
- Henson MC, Swan KF, O'Neil JS. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet Gynecol 1998;92:1020-1028.
- Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. J Clin Endocrinol Metab 2000;85:1946-1950.
- Duggal PS, Weitsman SR, Magoffin DA, Norman RJ. Expression of the long (OB-RB) and short (OB-RA) forms of the leptin receptor throughout the oestrous cycle in the mature rat ovary. Reproduction 2002;123:899-905.
- Smith JT, Waddell BJ. Leptin receptor expression in the rat placenta: changes in ob-ra, ob-rb, and ob-re with gestational age and suppression by glucocorticoids. Biol Reprod 2002;67:1204-1210.