One should ask a question of whether respiratory rehabilitation is at all possible in patients with interstitial lung diseases. In this study we attempted to address this issue by examining the efficiency of pulmonary rehabilitation in patients suffering from interstitial lung diseases, using the widely accepted questionnaires for the evaluation of the quality of life and the degree of perceived dyspnea in pulmonary fibrosis and COPD patients.
The research was approved by a Bioethical Commission of the Silesian Medical Academy (Permit No. NN-6501-244/04). All patients participating in the study were informed about the aim of the proposed rehabilitation program and gave written consent to participate in the program.
Patients
Thirty eight patients treated for interstitial lung disease in the years 2003-2004 in the Department of Lung Diseases and Tuberculosis of an academic hospital in the city of Zabrze, Poland were enrolled into the study. The group matched the following criteria:
- interstitial lung disease was diagnosed on the basis of radioclinical criteria (7, 8)
- patients reported at least 2 years of disease symptoms
- patients did not require home oxygen therapy
- patients were able to perform exercises on a bicycle ergometer
- treatment with no more than 20 mg of prednisone per day
- patients were at a stable stage of disease, free of infection or exacerbation of disease that would necessitate an increase in the corticosteroid dose
- 21 patients with idiopathic interstitial pneumonia
- 13 patients with idiopathic pulmonary fibrosis
- 8 patients with nonspecific interstitial pneumonia
- 4 patients with pulmonary fibrosis due to allergic alveolitis (chronic form)
- 5 patients with pulmonary fibrosis due to mix collagenosis
- 1 patient with pulmonary fibrosis due to silicosis
The rehabilitation program was composed of 4 weeks of rehabilitation held in the hospital and later continued by patients themselves at home. The exercise program was formed on the basis of the American Society (4), the British Thorax Society (5), and the American Society of Cardiologic and Respiratory Rehabilitation (6) recommendations. These recommendations were basically made out for COPD patients, but they may also be applied to patients with other respiratory disorders. Since the progress of pulmonary fibrosis is more dynamic than that of COPD, we decided to introduce a shorter, more dynamic, and individualized rehabilitation program that would take into account skills of each patient. The patients were informed about the rules and aims of the planned program. A diary was provided in which they were obliged to note any deviations from, or problems with, the execution of the exercise program. In the hospital environment, the program was introduced to the patient under the supervision of an experienced instructor of rehabilitation, and exercise was preceded by instruction and demonstration of the planned tasks.
The timing and intensity of the exercise program was prepared individually for each patient. The program consisted of general exercise, performed twice a week for 30 min, (movements of the thorax, correctional exercise, isometric exercise), respiratory muscle exercise, consisting of 6 series of 5-breath cycles interspersed with 1-min rest periods (altogether 30 breaths), run on Threshold IMP produced by Healthdyne Technologies (UK), and bicycle ergometer training, performed once a day for 15 min with a pretested 60% max load in Watts.
The level of perceived dyspnea and the quality of life were estimated before and after 6 weeks of rehabilitation. Dyspnea was evaluated using the following methods:
- Medical Research Council dyspnea scale (MRC) (9)
- Oxygen Cost Diagram (OCD) (10)
- Baseline Dyspnea Index (BDI) (10)
- Borg scale (11)
For the quality of life, SF-36 questionnaire (12) - generally describing Quality of Life - and St. George's Respiratory Questionnaire (13) - characteristic for chronic lung diseases - were used. The SF-36 consists of 36 questions that include basic domains describing the condition of health: Physical Functioning (PF), Role Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role Emotional (RE), and Mental Health (MH). Methodological rules and analysis of data by SF-36 questionnaire was described in a previous work (14). St.George's Respiratory Questionnaire consists of 50 questions grouped in 3 domains: symptoms, activity, and influence on life. Each domain contains an experimentally set number of points. The points that are responded to are pooled together and divided by the maximum number of points in each domain. The received score alternates between 0 (minimum handicap) and 100 (maximum handicap). Simultaneously a global outcome of the questionnaire was also counted. The agreement from Prof. J. Malolepszy, the owner of copyrights of the Polish version of St .George Questionnaire, to use the tool was obtained.
Data analysis
Data are expressed as means ±SD. Student's t-test, non-parametric Mann-Whitney test, and Fisher's Chi2 test were used for statistical comparisons. P<0.05 was considered significant.
Of the 38 patients with pulmonary fibrosis qualified to rehabilitation, 31 (81.6%) completed the program. All those who completed the program showed a great engagement in its realization. An analysis of individual diaries showed that the exercise program was executed, on average, in 91%. Fourteen of the 31 patients who completed the program realized it in 100% and the remaining in 80-98%. The most common reason for not performing the exercise properly was lack of free time, not health problems.
Dyspnea evaluation
The mean group score in the MRC questionnaire, before rehabilitation, was 2.3 ±0.8 (Table 1). The maximum dyspnea of 4 (on a 5 grade MRC scale) was declared by 2 patients (6.3%). Dyspnea of this intensity enables one leaving his house. Eleven patients (34.4%) declared dyspnea of 3rd grade, 14 (43.8%) of 2nd grade, and only 5 patients (15.6%) had dyspnea of 1st grade, which occurred with a bigger effort. After rehabilitation, the mean group MRC score decreased to 2.0 ±0.9 (Table 1), which barely missed statistical significance. However, 13 patients (40.6%) declared a lessening of dyspnea, 15 patients (46.8%) reported no change, and just 4 others (12.5%) reported deterioration.
The mean group OCD result, before rehabilitation, amounted to 72.2 mm (Table 1). None of the patients reported total lack of dyspnea (100 mm), 6 patients (18.8 %) marked vector 95 mm, which corresponds approximately to dyspnea occurring during an energetic uphill walk, 15 patients (46.9%) marked vector 75 mm, which corresponds to dyspnea occurring while making a moderated effort, 8 patients (25.0%) marked vector 60 mm, which corresponds to dyspnea occurring during a slow uphill walk, and 3 others (9.4%) marked vector 45 mm, which corresponds to dyspnea occurring while making bed. After rehabilitation, the mean group OCD result increased to 77.2 mm (Table 1); a trend for improvement in perceived dyspnea which did not reach statistical significance. Twenty patients (64.5%) declared improvement in dyspnea, 3 of whom reported an increase over 30 mm in the OCD diagram. Eleven other patients declared a worsening that in 4 patients amounted to a 15-30 mm decrease.
| Table 1. Scores of dyspnea severity before and after 6 weeks of pulmonary rehabilitation in patients with interstitial lung diseases. |
 |
| Values are means ±SD. MRC - Medical Research Council dypnea scale; OCD - Oxygen-cost diagram; FI - functional impairment - component of Baseline Dyspnea Index (BDI); MT - magnitude of task - component of Baseline Dyspnea Index; ME - magnitude of effort - component of Baseline Dyspnea Index; FI+MT+ME - total sum of BDI; Borg scale score. |
Before rehabilitation, lack of the ability to work and giving up all or almost all activities due to dyspnea (response 1 or 0) was reported by 5 of the examined patients (15.6%) in the FI/BDI questionnaire. The occurrence of dyspnea with easy activities such as washing, standing (response 1 in MT/BDI) was reported by another 5 patients and 1 patient reported dyspnea while sitting or lying (response 0 in MT/BDI). Seven patients (21.8%) reported that even a light effort causes dyspnea (response 1 in ME/BDI). Two patients (6.3%) reported the appearance of dyspnea while performing everyday activities. They marked 4 in FI/BDI. Three others (9.4%) estimated their initial ability to perform tasks as being good with dyspnea appearing under a greater strain only (point 4 in ME/BDI). The remaining patients marked point 2 or 3 in the BDI FI/MT/ME questionnaires. After rehabilitation, 4 patients marked 0 or 1 in FI/BDI, none marked 0, and 4 patients marked 1 in MT/BDI, and 6 others marked 1 in ME/BDI. The number of patients who felt some relief from dyspnea (answer number 4) increased to 4 patients in FI/BDI, 6 patients in MT/BDI, and 5 patients in the ME/BDI questionnaire. The mean group for all the answers (FI+MT+ME) increased from 6.3 before to 6.8 after rehabilitation, but the increase did not achieve statistical significance (Table 1).
The mean group score for dyspnea on the Borg scale, before rehabilitation, amounted to 3.0 (Table 1). The strongest dyspnea (6 on the 10-degree scale) was declared by 2 patients, 3 patients declared dyspnea of 5, 5 patients of 4, 7 patients of 3, 10 patients of 2, and the remaining 5 patients of 1. After rehabilitation, none of the patients declared dyspnea of 6, 4 patients declared dyspnea of 5, 4 patients of 4, 5 patients of 3, 10 patients of 2, and 9 others of 1. In all, 16 patients noticed improvement in perceived dyspnea after completion the rehabilitation program, 12 patients did not register any change, and 4 patients reported deterioration of dyspnea. The mean group score dropped to 2.5, which was a significant improvement (Table 1).
Quality of life evaluation
The mean scores of components of the SF-36 questionnaire in patients with interstitial lung diseases before and after rehabilitation are illustrated in Fig. 1. Rehabilitation caused the scores of all components to increase. The increases were significant for the majority, except the RP, BP, GH, and RE components. However, all the score increases were by <20 points, which makes them inessential from the clinical standpoint.
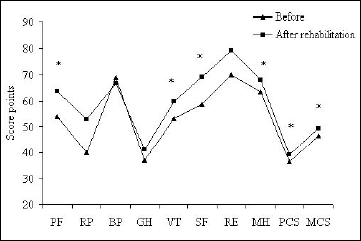 |
Fig. 1. Mean values of SF-36 Questionnaire components in interstitial lung disease patients before and after a 6-week pulmonary rehabilitation program. *P<0.05 for differences between the corresponding components before and after rehabilitation. |
Fig. 2 illustrates changes in the three domains of St. George's Respiratory Questionnaire under consideration: symptoms, activities, and influence on life resulting from the rehabilitation program. A global score of the questionnaire results also is presented. Essential improvements, statistically (P<0.03) and clinically (>4 points) significant were found in the activity (7.8 points), influence on life (8.3 points) as well as in the global outcome of the questionnaire (4.8 points).
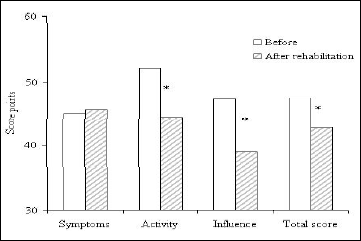 |
Fig. 2. Mean values of St. George's Respiratory Questionnaire components in interstitial lung disease patients before and after a 6-week pulmonary rehabilitation program. *P<0.03 for differences between before and after rehabilitation. |
Pulmonary rehabilitation in chronic pulmonary diseases has well defined goals, such as reductions in symptom intensity, exacerbations, hospitalizations, and improvements in motor activity and psychosocial performance of the patient, with an overriding aim to prolong the patient's life and make it of higher quality (15). Although these effects have been best described for patients with COPD, the results of the present study allow to state that some of basic tasks of rehabilitation are possible to achieve also in patients suffering from interstitial lung disease.
Dyspnea is supposed to be the most troublesome symptom in respiratory systemic diseases (16). In the present study, resting dyspnea was evaluated in patients with interstitial ling disease with a number of tools, such as the Borg scale, the MRC dyspnea questionnaire, the OCD diagram, and the BD index. After 6 weeks of rehabilitation, patients reported improvement in perceived dyspnea in all the scales used, although a statistically significant effect was seen only in the Borg scale. Lack of statistical differences in the other scales may be explained by the character of tools used for the evaluation of dyspnea. The MRC scale is one of the oldest dyspnea scales, created by Fletcher in 1952. The scale contains only 5 points, which makes it rather insensitive in the evaluation of clinical changes. Low sensitivity of this scale is in accord with the data of de Torres et al (17), who reported that clinically recognizable effects of pulmonary rehabilitation are noted in 29% of patients examined with the MRC scale, whereas the percentage of positively responding patients increases to 48% and more than 50% of those examined with a visual-analog and BDI scales, respectively. Similar limitations concern the OCD diagram, which is fairly easy to perform and evaluate, but dyspnea rather poorly reflects physical activity that requires increased oxygen consumption, as the scale does not fully account for, e.g., the speed with which the activity is performed. The BDI and Borg scales do not have these disadvantages. The BDI scale is quite detailed, as it contains 15 questions grouped in 3 domains estimating the degree of functional impairment, the kind of performed tasks, and the level of effort. Likewise, the Borg scale allows a precise determination of changes in the feeling of dyspnea the patient perceives. Recently, the BDI scale has been described as a useful tool in the estimation of rehabilitation programs by Mahler et al (18). In the present study, the number of patients who reported functional and physical improvements of >1 point on the BDI scale grew to 40%. This result is similar to that presented by Witek et al (19) from a multicenter evaluation of COPD patients who positively responded to 6-months treatment with salbutamol or ipratropium. Although patients with pulmonary fibrosis describe dyspnea in a different way than do those with COPD (20), the feeling not only correlates with the clinical course, but also is a key prognostic factor concerning mortality (21, 22).
Aside from decreasing the symptom severity, effective pulmonary rehabilitation should lead to a better function of the whole organism. Therefore, estimation of the quality of life is an inseparable element of respiratory rehabilitation. The SF-36 Questionnaire has been used by Martinez (23) to estimate a general quality of life in patients with interstitial pulmonary fibrosis. Our present evaluation of interstitial lung disease patients, using the SF-36, corresponds closely to results of that author. Our values, before rehabilitation, were different from those of Martinez (23) only in two domains, General Health (37.2 vs. 53.5, respectively) and Physical Functioning, which was estimated by our patients as being better (54.2 vs. 42.7, respectively); these differences are not clinically essential, as they do not exceed 20 points. There are no other data for the Polish population of interstitial lung disease patients to compare with, but the present results indicate that such patients estimate Bodily Pain and Emotional Health in the SF-36 Questionnaire best. In contrast, in an American population of such patients, Chang et al (24) demonstrated low values in the Physical Health and Physical Functioning domains. However, there are no available data on how the SF-36 results change after a course of pulmonary rehabilitation in interstitial lung disease patients. Such data exist with respect to COPD patients. Boueri et al (25) showed that a short 3-week rehabilitation program essentially improves the quality of life, particularly in the domains of Physical Functioning, Mental Health, and Physical Health. Those results, obtained in COPD, bear resemblance to the results of the present study obtained in interstitial lung disease patients.
St. George's Questionnaire, employed in the present study, showed more dedicated (>4 points), positive clinical effects of rehabilitation in interstitial lung disease patients. We found significant improvements in activity, influence on life, and total outcome in the questionnaire. There was no improvement in the domain concerning symptoms, which may be explained by the presence of questions specifically designed for COPD symptoms, such as caught, expectoration, whistling rales, and breathlessness attacks; the symptoms not be typically expected in pulmonary fibrosis. Lack of questions directly pertaining to pulmonary fibrosis patients may be one reason that such patients, despite worse results in functional respiratory tests, score better on this questionnaire than COPD patients do (26). For the same reason, the value of St. George's Questionnaire in the assessment of the quality of life of interstitial lung disease patients is questioned by some authors (27, 28), although the questionnaire, due to the dichotomic character of questions, has high sensitivity in unraveling even small clinical changes and is deemed excellent in the assessments of rehabilitation effects in COPD patients (29). Wedzicha et al (30) evaluated the results of an 8-week rehabilitation program in COPD patients, divided into subgroups of heavy and moderated dyspnea. Comparing the present study with the data of those authors, one can state that, for the same level of perceived dyspnea, patients with pulmonary fibrosis better respond to pulmonary rehabilitation than patients with a heavy form of COPD.
We conclude that patients with pulmonary fibrosis react positively to a 6-week pulmonary rehabilitation program, as estimated by dyspnea scales and questionnaires describing the quality of life. The introduction of rehabilitation programs for this category of pulmonary patients is thus worth giving further consideration.
- Lacasse Y. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996; 348: 1115-1119.
- Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 3: CD003793.
- Salman GF, Mosier MC, Beasley BW, Calkins DR. Rehabilitation for patients with chronic obstructive pulmonary disease: meta-analysis of randomized controlled trials. J Gen Intern Med 2003, 18, 213-221.
- American Thoracic Society. Pulmonary rehabilitation - 1999. The Official Statement of The American Thoracic Society. S Lareau, R Zu-Wallack, B Carlin (eds). Am J Respir Crit Care Med 1999, 159: 1666-1682.
- Pulmonary rehabilitation. BTS Statement. Thorax 2001; 56, 827-834
- Pulmonary Rehabilitation. Joint ACCP/AACVPR Evidence-Based Guidelines. Chest 1997; 112: 1363-1395.
- Baughman R, Du Bois RM, Lynch JP, Wells AU. Diffuse Lung Disease. A Practical Approach. London, Arnold. 2004.
- Idiopathic Pulmonary Fibrosis: Diagnosis and Treatment. International Consensus Statement. Am J Respir Crit Care Med 2000; 161: 646-666.
- Fletcher CM. Standardized questionnaire on respiratory symptoms: A statement prepared and approved by the MRC Committee on the aetiology of chronic bronchitis (MRC breathlessness score). BMJ 1960; 2: 241-243.
- Baddini Martinez JA, Martinez TY, Lovetro Galhardo FP, de Castro Pereira CA. Dyspnea scales as a measure of health-related quality of life in patients with idiopathic pulmonary fibrosis. Med Sci Monit 2002; 8: CR405-410.
- Borg GAV. Psychophysical basis of perceived exertion. Med Sci Sports Exerc 1982; 14: 377-381.
- Ware JE, Kosinski M, Gandek B. SF-36 Health Survey. Manual & Interpretation Guide. QualityMetric Incorporated, Lincoln, 2004.
- Kuzniar T, Patkowski J. Kwestionariusz Szpitala Sw. Jerzego (St. George's Respiratory Questionnaire) jako narzedzie oceny jakosci zycia w chorobach ukladu oddechowego. Pol Arch Med Wewn 2000; IV, 1: 401- 406.
- Jastrzebski D, Kozielski J, Banaś A et al. Quality of life during one-year observation of patients with idiopathic pulmonary fibrosis awaiting lung transplantation. J Physiol Pharmacol 2005, 56 Suppl 4: 99-106.
- Ferrer M, Villasante C, Alonso J et al. Interpretation of quality of life scores from the St George's Respiratory Questionnaire. Eur Respir J 2002; 19: 405-413.
- Pashkow P, Ades PA, Emery CF et al. Outcome measurement in cardiac and pulmonary rehabilitation. J Cardiopulm. Rehabil 1995; 15: 394-405.
- de Torres JP, Pinto-Plata V, Ingenito E et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest 2002; 121: 1092-1098.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnoe. Chest 1988; 93: 580-586.
- Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnea index in a multinational clinical trial. Eur Respir J 2003; 21: 267-272.
- Mahler DA. Description of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med 1996; 154: 1357-1363.
- Mahler DA, Harver A, Rosiello R et al. Measurement of respiratory sensation in interstitial lung disease: Evaluation of clinical dyspnea ratings and magnitude scaling. Chest 1989; 96: 767-771.
- American Thoracic Society. Dyspnea. Mechanisms, assessment and management: A consensus statement. Am J Respir Crit Care Med 1999; 159: 321-340.
- Martinez TY. Evaluation of the Short-Form 36-item Questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest 2000; 117: 1627-1632.
- Chang JA. Assessment of health-related quality of life in patients with interstitial lung disease. Chest 1999; 116: 1175-1182.
- Boueri FM, Bucher-Bartelson BL, Glenn KA, Make BJ. Quality of life measured with a generic instrument (Short Form-36) improves following pulmonary rehabilitation on patients with COPD. Chest 2001; 119: 77-84.
- Ketelaaes CA, Schlosser MA, Mosteri R et al. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax 1996; 51: 39-43.
- Griffiths TL, Burr ML, Campbell IA et al. Results at 1 year of multidisciplinary pulmonary rehabilitation: A rondomised controlled trial. Lancet 2000; 355: 362-368.
- De Vries J, Kessels BLJ, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J 2001; 17: 954-961.
- De Vries L. Measuring quality of life in interstitial lung disease. Chest 2000; 118: 275.
- Wedzicha JA, Bestall JC, Garrod R et al. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998; 12: 363-369.