CHARACTERIZATION AND BIOLOGICAL EFFECTS OF HYPERICUM EXTRACTS
ON EXPERIMENTALLY-INDUCED – ANXIETY, OXIDATIVE STRESS
AND INFLAMMATION IN RATS
INTRODUCTION
Anxiety disorders are currently the most prevalent psychiatric diseases in Europe and in the USA, thus representing a major issue for public health (1). In the past decades, scientists have mainly focused on γ-aminobutyric (GABA) and serotoninergic systems as a cause of anxiety disorders (2). But, lately, there is growing evidence, that oxidative stress imbalance and immune system alterations may be associated with the development of neuropsychiatric disorders, such as anxiety and depression (3, 4).
It is well known that low concentrations of reactive oxygen species (ROS) are essential for physiological processes, such as cellular signaling involved in plasticity process, immune defense mechanisms, apoptosis and cellular senescence (5). However, overproduction of reactive oxygen species (ROS) can damage cell macromolecules (lipids, proteins, DNA) and alter brain function (6).
Central nervous system (CNS) is considered to be highly vulnerable to oxidative damage, due to its high content of lipid molecules, large oxygen consumption and its relatively modest antioxidant capacity (7). Moreover, in the brain, the metabolism of catecholamines and of neurotransmitters is also an important source of free radical production (8). Therefore, oxidative stress is one possible mechanism that may be related to inflammation and anxious behavior. Clinical and experimental studies indicate that anxiety is associated with high levels of ROS, both in the peripheral blood (lymphocytes, granulocytes and monocytes) and in the brain (neuronal and glial cells in the cerebellum, hippocampus and neurons of the cerebral cortex) (2). In addition, scientific evidences sustain that stress can activate the inflammatory cascade, fact demonstrated by elevated serum and brain concentrations of pro-inflammatory cytokines in rodents such as tumor necrosis factor-α (TNF-α), interferon-c (IFN-c), IL-1β, and IL-6. Cytokines, a group of polypeptide mediators, that classically regulate local immune/inflammatory responses, can trigger the activation of both the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system, resulting in the release of adrenal glucocorticoids (cortisol in humans and corticosterone in rodents) (6). Thus, plasma or serum corticosterone can be utilized as a systemic marker of stress (9). Additionally, elevated levels of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 could serve as biological markers of anxiety disorders.
The NFκB (nuclear factor κ-light-chain-enhancer of activated B cells) has long been considered a proinflammatory signaling pathway, due to its involvement in cytokine, chemokines, and adhesion molecules production, but lately, based on its apoptotic function, NFκB is believed to exert an anti-inflammatory function as well (10).
NFκB is activated in response to either stress, cytokines free radicals, ultraviolet irradiation, oxidized LDL, bacterial and viral infections, or nerve growth factor and glutamate, that are considered brain-specific activators of NFκB (11). The role of NFκB in the nervous system is involved in synaptic processes, neurotransmission, and neuroprotection, thus NFκB being associated with synaptic plasticity (12). Based on the above mentioned information, such as inflammatory process regulator, and synaptic plasticity, development and neuron survival modulator, we evaluated the expression of NFκB and pNFκB in whole homogenates of hippocampus and frontal lobe.
The efficiency of anxiolytic drugs is well known, however, their administration may lead to numerous side effects (sedation, dependence, rebound anxiety, discontinuation syndrome, cognitive, and psychomotor impairment) (13). Considering the multiple hypotheses regarding the mechanism that leads to anxiety and the side effects of classical medication, the need for an alternative therapy that may provide some symptomatic relief is highly needed.
Quercetin (3,3’,4’,5,7-pentahydroxyflavone), is a common flavonol found in fruits, vegetables, leaves and grains with neuroprotective effects. As an antioxidant effect, quercetin may stimulate cellular defenses against oxidative stress by induction of Nrf2-ARE factor and paraoxonase 2 (PON2) activity and may inhibit lipid peroxidation and stimulate mitochondrial biogenesis. As a neuroprotective effect, quercetin can activate sirtuins (SIRT1), induces autophagy and acts as a phytoestrogen (14).
The genus Hypericum L. (Hypericaceae) is represented by twelve species in Romanian spontaneous flora and H. perforatum L. and H. maculatum Crantz are considered the most widespread (15).
Hypericum perforatum is a perennial plant, with worldwide distribution, commonly known as St. John’s wort, recognized for their pharmacological properties: wound healing anti-inflammatory, antiviral, antimicrobial and antioxidant and antitumoral effects. Hypericum perforatum has also been used for the symptomatic treatment of mild-to-moderate depression and lately for the treatment of anxiety (16).
Despite all the aforementioned beneficial effects of Hypericum sp., the neuroprotective, and neurochemical effects of Hypericum perforatum in comparison to H. maculatum on FG 7142 experimentally - induced anxiety, were never studied before. Additionally, based on the flavonoids constituents of Hypericum sp. we choose quercetin as a positive control for this study.
Based on these data, our study aimed to evaluate the effect of some isolated natural compounds (quercetin, Q) or constituents of some phytocomplex such as of Hypericum maculatum (HM) and Hypericum perforatum (HP) extracts on brain oxidative stress biomarkers, inflammatory cytokines and serum corticosterone levels. NFκB signaling pathway in the hippocampus and frontal lobe in rats with FG-7142 experimental-induced anxiety were also investigated.
MATERIALS AND METHODS
Reagents
FG-7142 (FG) in 2-hydroxypropyl cyclodextrin (40%w/v) was obtained from Sigma-Aldrich Chemicals GmbH (Germany). Alprazolam (APZ) (0.5 mg) was purchased from Terapia Ranbaxy, Cluj, Romania. 2-thiobarbituric acid and Bradford reagent were obtained from Merck KGaA (Darmstadt, Germany). ELISA tests for cytokines were purchased from Signosis, Inc., Santa Clara, California, and for serum and plasma corticosterone was bought from IBL-International, Hamburg, Germany. The dry extracts were dissolved in 0.5 ml of carboxymethylcellulose (CMC, 2%, from Sigma-Aldrich). Hypericum perforatum dry extract was commercialized by Naturex France®. The hypericin reference standard was from Roth, Germany (HPLC grade) and the hyperforin and quercetin were obtained from Sigma-Aldrich Chemicals GmbH (Germany). All chemicals and reagents were of high-grade purity.
Dry extract obtaining and analysis
There were analyzed 2 dry extracts. One of them was commercialized by Naturex France® and was obtained from Hypericum perforatum using ethanol 30% and extract ratio 10/1. The second extract was obtained from Hypericum maculatum by the following protocol: aerial part of H. maculatum plant was harvested from wild populations from Cluj County (area: 46° 45’ N, 22° 47’ E) at a full flowering stage in June 2016. The voucher specimen (No. 452) were taxonomically identified and deposited in the Herbarium of the Pharmacognosy Department, Faculty of Pharmacy, “Iuliu Haţieganu” University of Medicine and Pharmacy Cluj-Napoca. For tincture preparation, the vegetal material was air-dried, reduced to a powder and then extracted by maceration with ethanol 70%, 10 days, at room temperature, vegetal material/solvent ratio was 1/5 (17).
For dry extract preparation the tincture was dried using the fluid bed drying method and an Aeromatic Strea 1 apparatus (GEA, Switzerland). H. maculatum tincture was adsorbed on a powder mixture containing lactose and cellulose (1:3) (15). Phytochemical screening on both dry extracts was made by using qualitative and quantitative chemical methods. The chemical analyses of total hypericins were performed by spectrophotometric analysis and were expressed in hypericin (%, g/g dry extract) and hypericin, hyperforin and polyphenols derivatives were quantified by chromatographic methods by using a Jasco V-530 UV-vis spectrophotometer (Jasco International Co., Ltd., Tokyo, Japan) (18).
High-performance liquid chromatography samples preparation and analysis
Quantification of main compounds was performed by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS). The high-performance liquid chromatography (HPLC) system used was an 1100 series Agilent Technologies model (Agilent, Santa Clara, CA, USA) coupled with an Agilent Ion Trap Detector 1100 SL equipped with electrospray ionization (ESI) system, a binary pump (G13311A), an in-line degasser (G1322A), an autosampler (G1313A), a column thermostat, and UV detector (G1316A).
Determination of hypericin and hyperforin content
An aliquot (obtained from1 g extract with 10 mL ethanol 70%, sonicated in an ultrasound bath for 3 hours) was injected into the chromatographic system. Chromatographic separation was performed on a Zorbax SB-C18 (50 mm × 2.1 mm i.d., 3.5 µm) column (Agilent Technologies, USA) with a mixture of acetonitrile/1 mM ammonium acetate in water, 50/50 (v/v), at 45ºC with a flow rate of 0.6 mL min–1. The elution was performed as described elsewhere (19). Hypericin and hyperforin were identified using retention time (RT) of 1 and 2.5 min, respectively. These compounds were detected in the non-reactive MS2 mode for hypericin or in reactive MS2 mode for hyperforin, with negative ion ionization, using electrospray ionization (ESI) ion source. Quantification of hypericin and hyperforin were made using the calibration curves with good linearity (R2 > 0.999) for five employed points plots (19).
High-performance liquid chromatography analysis of polyphenolic compounds
For the identification and quantification of polyphenols, the analysis was performed in previously described conditions using polyphenolic compounds standards. The standards used were: polyphenolic acids (caftaric, gentisic, caffeic, chlorogenic, p-coumaric, ferulic, sinapic acids), quercetin and their glycosides (hyperoside, quercitrin, isoquercitrin, rutin) and other flavonoid aglycon such as myricetin, fisetin, patuletin, luteolin, apigenin, kaempferol. The quantification was based on the calibration curves in the 0.5 – 50 µg/mL concentration range with good linearity (R2 > 0.999) for a five points plots were employed (20, 21). All the samples were analyzed in triplicate.
Animals and experimental design
Experimental procedures were approved by the Animal Ethics Board on animal welfare of “Iuliu Hatieganu” University and by the Direction for Veterinary Surveillance and Food Safety according to the Directive 2010/63/EU on the protection of animals used for scientific purposes (Authorisation No. 2/13.07.2016). Two-month-old male Wistar rats (n = 60) were used under standard laboratory conditions.
To evaluate the biological effects of quercetin (Q), Hypericum maculatum dry extract (HM) and Hypericum perforatum dry extract (HP) on brain oxidative stress biomarkers and inflammatory cytokines in rats with experimental-induced anxiety, the animals were divided into 6 groups of 10 rats each (Fig. 1). One group received CMC 2% and served as control (CMC). The animals in group 2 were given CMC and a partial inverse agonist of benzodiazepines (BZD), FG- 7142. The other groups of animals were treated with: APZ dissolved in CMC and FG (APZ + FG); Q dissolved in CMC and FG (Q + FG); HM dissolved in CMC and FG (HM + FG); HP dissolved in CMC and FG (HP + FG). APZ (0.08 mg/kg b.w.), Q (30 mg/kg b.w.), HM and HP (350 mg/kg b.w.) were orally administered for 21 days, dissolved in 0.5 mL carboxymethylcellulose, based on the published data of Trofimiuk et al. (22). Consequently, alprazolam (0.08 mg/kg) was chosen as the standard anxyolitic drug (23). FG (7.5 mg/kg b.w.) was intraperitoneally (i.p.) injected in a single dose (24). Three hours later, under anesthesia with an intraperitoneal injection of ketamine/xylazine cocktail (90 mg/kg b.w. ketamine and 10 mg/kg b.w. xylazine), all animals were euthanized. The hippocampus and frontal lobe from 5 rats in each group were harvested for the oxidative stress cytokines analysis and western blot technique. The whole brain from 5 rats in each group were harvested for histopathological investigations.
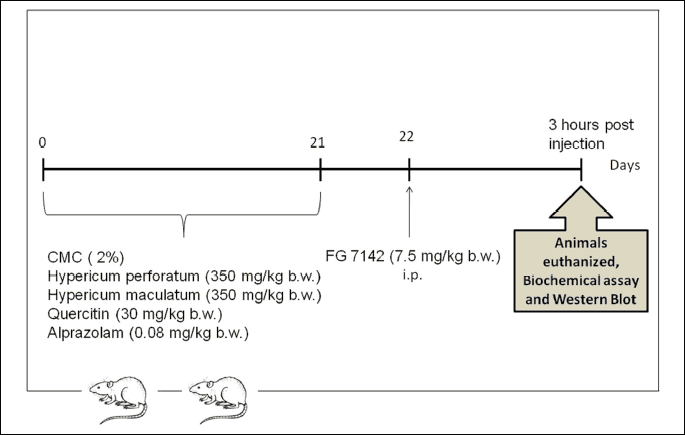
Biochemical investigations of oxidative stress
The malondialdehyde (MDA) levels were considered as lipid peroxidation marker, while catalase (CAT) and superoxide dismutase (SOD) activities were evaluated as antioxidant biomarkers. The MDA levels, CAT and SOD activity in hippocampus and frontal lobe were determined as described by Conti et al. (25), Pippenger et al. (26) and Fridovich (1989) (27).
Quantitative estimation of serum corticosterone, interleukin-1a, and -1b, RANTES, monocyte chemoattractant protein-1 and interferon levels by ELISA technique
Serum corticosterone level was measured using an ELISA kit for the quantitative in vitro diagnostic measurement of corticosterone in serum and plasma according to the manufacturer’s protocol (IBL-International, Hamburg, Germany). Results are expressed as nmol/L.
The concentration of cytokines was determined by ELISA (Rat Inflammation ELISA Strip for Profiling Cytokines) according to the manufacturer’s protocol (Signosis, Inc., Santa Clara, California). Results are expressed either as pg/mg protein or as OD/mg protein.
Evaluation of nuclear factor-κB/phospho-nuclear factor-κB expressions by Western blot
NFκB and phospho-NFκB quantification was performed by western blot technique (BioRad) by using antibodies against NFκB and phospho-NFκB (Ser536) (93H1) (Santa Cruz Biotechnology, Heidelberg, Germany). Proteins were visualized and detected using Supersignal West Femto Chemiluminiscent substrate (Thermo Fisher Scientific, Rockford IL, USA) and a Gel Doc Imaging system equipped with a XRS camera and Quantity One analysis software (Biorad). β-actin was used as a protein loading control.
Statistical analysis
All statistical analyses were conducted using ANOVA GraphPad Prism software, version 6.0 (GraphPad, San Diego, California, USA) and SPSS v.11.5 for Windows. The results were expressed as the mean ± standard deviation (SD). One way analysis of variance (ANOVA) was used, followed by Tukey’s post hoc test, to determine statistical significant among the four groups. One way analysis of variance (ANOVA) was used, followed by Bonferroni `s post hoc test, to determine statistical significant among two groups. A P-value lower than 0.05 was considered statistically significant. Related to extracts, differences between means were assessed by a Student-Newman-Keuls test (P ≤ 0.05). Each treatment contained three independent replicates and the experiments were performed twice.
RESULTS
The content of hypericin and hyperforin and high-performance liquid chromatography analysis of the extract
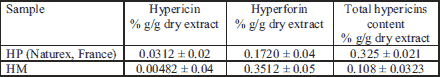
The content of hypericin and hyperforin revealed by HPLC method and of the total hypericins determined by the spectrophotometric method are presented in Table 1.
HPLC method has been developed for the determination of 19 standard polyphenolic compounds. The quantitative determination of phenolic compounds identified was performed using the external standard method. The concentrations of HPLC identified polyphenolic compounds in both dry extracts are presented in Table 2. They were shown in the order of their retention time.
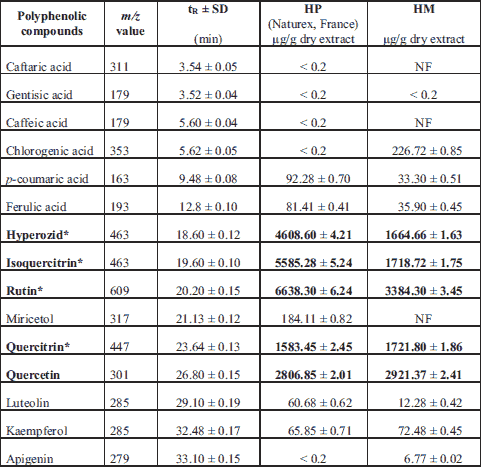
* Quercetin derivatives
Oxidative stress assessment in hippocampus and frontal lobe
The malondialdehyde (MDA) levels and catalase (CAT) activities in the different areas of brain, in rats pretreated with some natural compounds after FG administration were illustrated in Fig. 2A-2D.
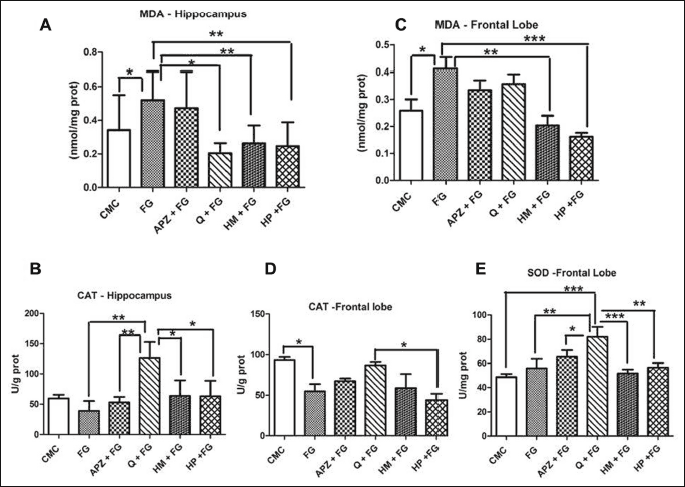
Quercetin (P < 0.05) and HP administration (P < 0.01) diminished the MDA levels in hippocampus, while HM (P < 0.01) and HP treatment (P < 0.001) decreased lipid peroxidation in frontal lobe. MDA displayed higher levels in the hippocampus and frontal lobe of FG group (P < 0.05). CAT activity were significantly lower in the FG group as compared to CMC in frontal lobe, (P < 0.05). In hippocampus, CAT activity decreased after FG treatment (P > 0.05) but the differences were not statistically significant. After 21 days of quercitin administration, CAT activity increased, both in hippocampus, as compared to HM and HP (P < 0.05) and in frontal lobe, as compared to HP (P < 0.05). In frontal lobe, quercitin improved SOD activity as compared to HM and HP (P < 0.05).
Quantitative estimation of serum corticosterone levels
The influence of natural compounds administration on corticosterone level in the serum was shown in Fig. 3. The anxiogenic drug significantly elevated the serum corticosterone levels as compared to CMC group (P < 0.01), while APZ (P < 0.001), Q (P < 0.05), HM (P < 0.001), and HP (P < 0.001) decreased its values as measured by ELISA.
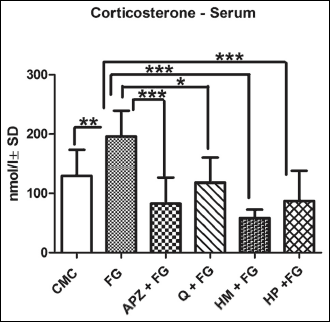 |
Fig. 3. Quantification of corticosterone in the serum in rats. The anxiogenic drug significantly elevated the serum corticosterone levels as compared to CMC group; APZ, HM and HP decreased its values as measured by ELISA. Each group consisted of 5 rats. Results are expressed as mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.001. |
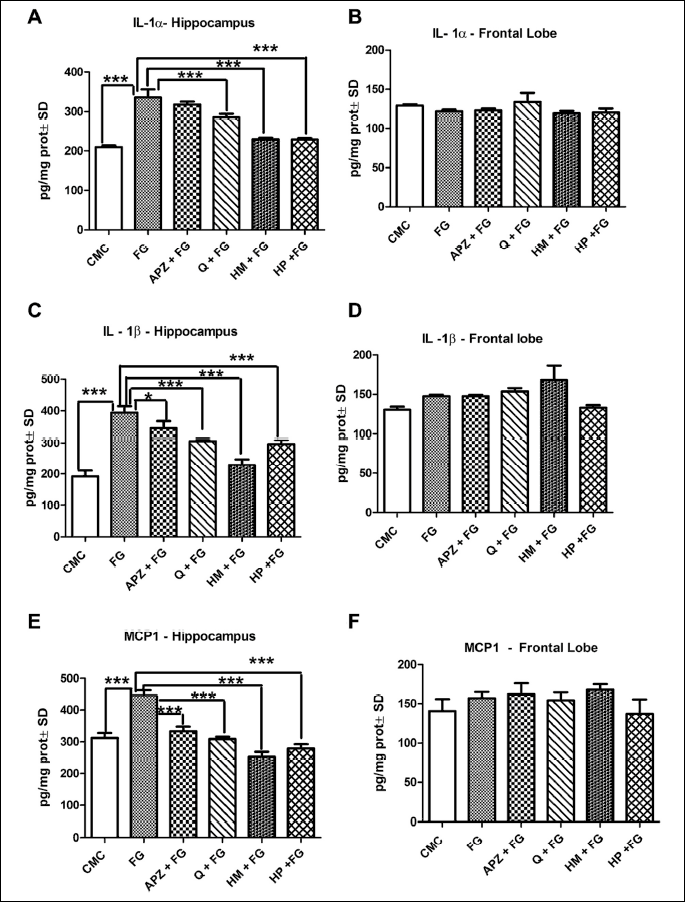
Quantitative estimation of brain interleukin-1a and 1b, RANTES, monocyte chemoattractant protein-1, macrophage inflammatory protein and interferon levels
The influence of natural compounds administration on proinflammatory cytokines levels in the brain was shown in Fig. 4 and 5. The hippocampus showed significant increases, in both IL-1α and IL-1β cytokines in the anxiogenic drug treated group as compared to CMC group (P < 0.001). Conversely, Q, HM and HP downregulated both IL-1α and IL-1β, in the hippocampus, as compared to FG (P < 0.001). APZ treatment was associated with a decrease in IL-1β levels only in the hippocampus (P < 0.05). In the frontal lobe, the cytokines displayed no statistical difference between the groups (Fig. 4A-4D).
Both MCP1 and IFN were upregulated by FG 7142 in the studied brain areas, even though in frontal lobe, the differences were not statistically significant. The hippocampus exhibited significant decreases of MCP1 in APZ, Q, HM and HP groups as compared to FG (P < 0.001). INF levels were downregulated only by Q and HM in frontal lobe (P < 0.001; P < 0.01). HP elevated the IFN levels as compared to HM (P < 0.001) in hippocampus, while in frontal lobe the same marker was significantly decreased by HP as compared to HM (P < 0.001).
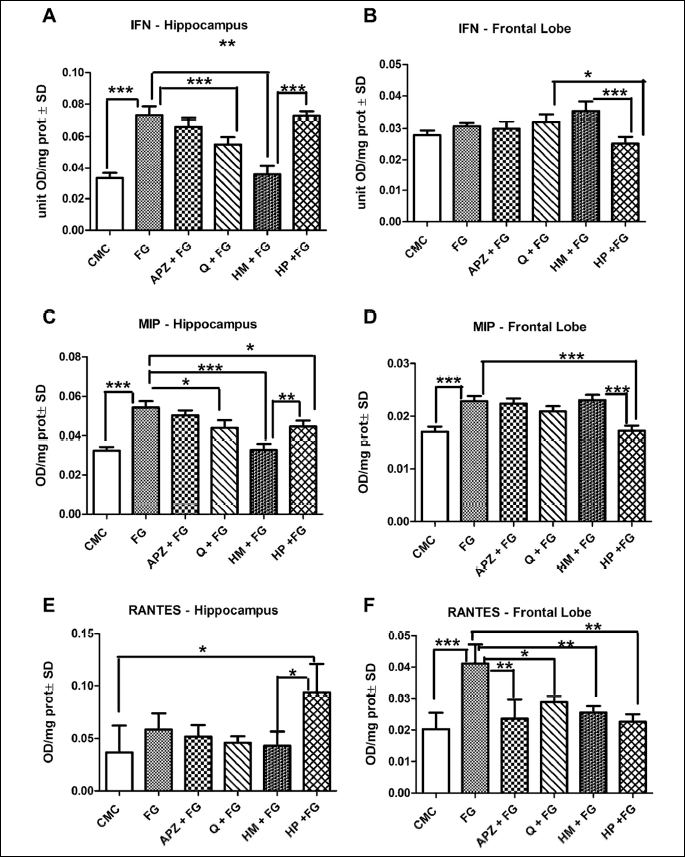
MIP recorded elevated levels both in hippocampus and frontal in the anxyogenic treated group as compared to CMC group (P < 0.001), while RANTES displayed higher values just in frontal lobe of the anxious - like rats (P < 0.001). Q, HM and HP down-regulated MIP levels in the hippocampus (P < 0.05; P < 0.001; P < 0.05). In the frontal lobe, RANTES values were significantly lower in APZ, Q, HM and HP groups (P < 0.01; P < 0.05; P < 0.01; P < 0.01). Cytokine, such as MIP (P < 0.01) and RANTES (P < 0.05) exhibited higher values in hippocampus of the HP treated rats in comparison to HM group. In the frontal lobe, both parameters decreased in HP treatment as compared to HM (MIP: P < 0.001; RANTES: P > 0.05) (Fig. 5A-5F).
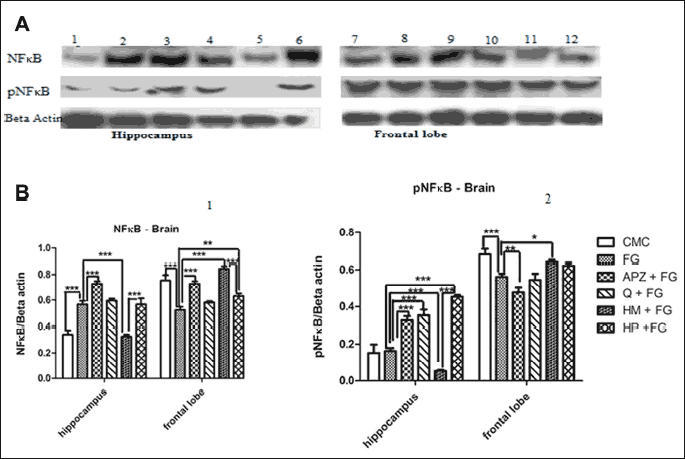
Quantitative estimation of nuclear factor-κB/phospho-nuclear factor-κB expressions in hippocampus and frontal lobe
The influence of natural compounds administration on NFκB/pNFκB expressions in the brain was exemplified in Fig. 6. FG 7142 significantly increased the NFκB level in hippocampus and decreased the expressions of NFκB and pNFκB (the active form) in frontal lobe as compared to control group (P < 0.001). Alprazolam enhanced the NFκB level, both in hippocampus and frontal lobe (P < 0.001) and pNFκB in hippocampus (P < 0.001). APZ also decreased the pNFκB value in frontal lobe (P < 0.001). Q significantly stimulated the active form of NFκB in the hippocampus (P < 0.001) while in the frontal lobe, HM administration increased the NFκB (P < 0.001) and pNFκB (P < 0.05) levels, but in the hippocampus HM lowered their values.
DISCUSSION
It is well known that HpE contain flavonoids (quercetin, hyperoside, rutin, isocvercitrin and quercitrin), naphtodiantrones (hypericin, pseudohypericin), and prenylated acylphloroglucinols (hyperforin and adhyperforin). Thus, besides the flavonoids, the other constituents of HpE are considered to be responsible for their beneficial effects, due to the multiplicity of bioactive compounds or of the synergistic action of those (16, 28).
In this study, we have chosen a pharmacological behavioral model, such as FG 7142 administration, to induce anxiety like behavior in rats. The anxiogenic drug, FG 7142, is a partial inverse agonist at the benzodiazepine allosteric site, with highest affinity for the α1 subunit-containing GABAA receptor. This compound modulates the GABA-induced chloride flux and interacts with the serotoninergic, dopaminergic, cholinergic, and noradrenergic modulatory systems of the anxiety-related neural network (29). Additionally, FG-7142 produces expression of c-Fos immunoreactivity in anxiety and stress -related brain regions (30). Consequently, alprazolam (0.08 mg/kg) was chosen as the standard anxyolitic drug (23).
In the present research, we provide evidence that FG 7142 administration increased the oxidative stress parameters in the brain of the anxious rats. These observations are consistent with the literature. Several studies reported enhanced protein carbonyl content in prefrontal cortex, high MDA levels in hippocampus and amygdale, and increased plasma 8-isoprostane, as well as altered enzymatic activity (increased activity of glutathione reductase 1 and glyoxalase 1) in the brain of the anxious mice (2).
It is well known that the brain is highly susceptible to oxidative stress due to its rich polyunsaturated fatty acids content, low antioxidant capacity and increased metabolic rate. Recent studies suggested that oxidative stress (6), mitochondrial dysfunction (31) and inflammation (32) may play an important role in psychiatric and neurodegenerative disorders. So, quercitin along with both extracts of Hypericum were chosen to be studied in this experimental model due to their beneficial effects.
Their actions are due to the presence of hypericin, flavonoids annin, acylphloroglucinol, essential oils etc. Hypericins and hyperforins are the most important active principles for antidepressant activity and the flavonoids may increase the pharmacological activity of other chemical compounds also. The results of the present study showed that 21 days of quercetin administration diminished the lipid peroxidation and improved the antioxidant activity in hippocampus of the FG-treated rat. The effect of anxiogenic drug was prevented by HM and HP treatment in frontal lobe and by HP in hippocampus. These observations confirm that impaired antioxidant activity and increased oxidative stress in neurons are involved in pathological mechanisms of different neuropsychiatric conditions (3).
Sanchez-Reus et al., (33) revealed that Hypericum perforatum extract reversed the redox misbalance induced by rotenone in rats’ brain, thus demonstrating the antioxidant and protective activity of this compound. Inhibition of lipid peroxidation was due to extract’s scavenging effect on NADPH and Fe2C/ascorbate generated free radicals (34). Hypericum perforatum exhibited antioxidant activity by either lowering NO content in axotomized retinas (16). or by inhibiting xanthine oxidase activity in a dose-dependent manner (35).
The antioxidant mechanism of HP extract may be related to quercitin, the most potent scavenger of ROS, RNS and peroxynitrite identified in the flavonoid family (16). Based on the quantitative determinations of polyphenols, our dry HP extract revealed a higher content of quercetin and quercetin derivatives as compared to the dry extract of HM, this being in agreement with the above mentioned data. Considering all the aforementioned data, it seems that several mechanisms and a multiplicity of bioactive compounds are responsible for the neuroprotective activity of HpE.
As oxidative stress is reported to trigger neuroinflammation, the levels of proinflammatory cytokines were examined. We observed that the anxiogenic drug rise in IL-1α, IL-1β secretion in hippocampus was prevented by Q, HM and HP administrations. So, indeed, we reached similar conclusions, regarding the anti-inflammatory effect of quercitin and Hypericum sp. extract, to those mentioned in the literature (6).
Conversely, the levels of proinflammatory cytokines were influenced neither by FG 7142 drug, nor by the natural compounds in the frontal lobe. The various responses in between the brain regions may be due to the different types of cytokines produced in brain and to their various functions. There is scientific evidence that immune dysregulations may consistently associate with psychiatric disorders. Although, many studies have shown overexpression of inflammatory markers, such as C-reactive protein (CRP), pro-inflammatory cytokines, including IL-1α, IL-2, IL-2, IL-3, IL-6, IL-5, IL-8, IL-9, IL-12A, IL-13, IL-15 IL-18, IFN-γ, TNF-α, MCP1, MIP 1α, and RANTES in depressed persons, lately some authors mentioned that immune dysregulations may also play a role in anxiety. Accordingly, it is mentioned that anxiety symptoms are associated with increased cerebral levels of proinflammatory cytokines, IL-1β, TNF-α (8). The authors mentioned that cytokines secretion, such as IL-6, IL-8, IL-10, IL-18, MCP-1, MMP2, TNF-β, was rather associated with anxiety symptoms than with depressive ones in lipopolysaccharide-stimulated inflammation.
The exact link between inflammation and depression is not fully elucidated and sometimes the results are contradictory. There is evidence that, inflammatory cytokines (IL-1β, TNF-α, IL-3, IL-5) activate p38 mitogen activated protein kinase (p38 MAPK), which activates the serotonin transporter, thus increasing serotonin uptake and decreasing its synaptic availability. In addition, proinflammatory cytokines, e.g. IFN-γ, shift tryptophan, the precursor to serotonin to kynurenine synthesis, thus depletes the serotonin levels. Moreover, cytokines, such as IL-6 and TNF-α, breakdown 5-hydroxytryptophan (5-HT) into 5-hydroxyindoleacetic acid, therefore, reducing the amount of produced serotonin and increasing the amount of degraded serotonin (35).
As anxiety and depression are highly comorbid disorders, they might share similar etiology. So, a possible explanation for the benefic effects of quercetin on the anxiety-like behavior could be the antioxidant and the anti-inflammatory effects of this compound. It is known that quercetin modulates inflammation by inhibitory effect on cyclooxygenase (COX) and lipoxygenase (LOX) or by interference with production of pro-inflammatory (TNF-α and IL-17) and/or anti-inflammatory (IL-10) cytokine (36).
Several studies showed that serum cortisol levels are a reliable indicator of stress response both in humans and animals (37, 38). In the present study, the serum corticosterone levels were used as a complementary means of monitoring stress in FG-7142 injected rats. APZ, Q, HM and HP markedly reduced plasma corticosteron levels, thus demonstrating the favorable effects of antioxidants on stress parameter. Accordingly, our data were consistent with those of Haleagrahara et al. (39), who reported the benefits of quercetin on stress-induced oxidative damage in the brain of the rats. The link between the HPA axis and oxidative stress is highly sustained. However, corticosteroids effect on NOX-dependent ROS production is cell-type dependent. For example, glucocorticoids inhibited the generation of NOX-derived superoxide in phagocytes, superoxide production in human aortic smooth muscle, NOX-dependent ROS production in microglia and hippocampal neurons (4).
Previous studies revealed that proinflammatory cytokines (e.g. IL-1, IL-6, and TNF-α) can activate HPA axis, in vivo, in various species, fact that causes secretion of glucocorticoids. The release of GC is induced by cytokines action either via hypothalamic corticotropin-releasing hormone (CRH) or by direct action effect on pituitary and adrenal glands. Cytokines can also be synthesized in the brain, the anterior pituitary, and the adrenal gland, thus being able to amplify the HPA response (38) (40). Some mention the constitutive expression of cytokines in brain regions, both in humans and rodents, while, on the other hand, others report undetectable levels of cytokines under basal condition.
In addition, we evaluated the NFκB and pNFκB expressions in the hippocampus and frontal lobe. Nuclear factor kappa B NFκB is a ubiquitously expressed transcription factor that can be activated by viral and bacterial infection, UV light, ionizing radiation, free radicals, lymphokines and cytokine, neurotoxins, etc. Moreover, physiological stimuli, such as nerve growth factor and glutamate are also considered brain-specific activators of NFκB (41, 42).
In our study, one dose of FG 7142 increased the NFκB level in hippocampus. HM administration in rats treated with anxyogenic drug diminished expression of NFκB and activation of pNFκB in hippocampus. Conversely, in the frontal lobe, regarding HM administration, our results were contradictory with the above mentioned data. APZ administration increased the expression of NFκB both in hippocampus and frontal lobe and activation of pNFκB in hippocampus. HP exerted stimulatory effects on NFκB in frontal lobe and on pNFκB in hippocampus. These contradictory findings can be partially explained by various stimuli that have been demonstrated to activate NFκB in nervous system. The constitutive activity of NFκB was initially identified in glutamatergic neurons of the CNS, such as the cerebral cortex, hippocampus, amygdala, olfactory lobes, cerebellum, and hypothalamus, thus being suggested that constitutive NFκB is the results of synaptic activity (42).
It seems that constitutive NFκB activation in neurons is associated with processing of neuronal information, as being involved in translating short-term signals from distant sites in neurites into long-term changes in gene expression. This mechanism may explain constitutive NFκB activation involvement in synaptic plasticity, development and neuron survival as demonstrated by previous studies, while the inducible NFκB in glial cells is responsible of various inflammatory processes. Considering the fact that, in our study HM diminished the cytokines levels, the expression of NFκB and activation of pNFκB in hippocampus, the natural compound may have anti-inflammatory effect (43).
Conversely, in glial cells NFκB exhibits low basal activity, being highly inducible. Inducible NFκB activity might be linked to pathological events, such as regulation of inflammation-induced neurodegeneration (41, 42). Sarnico et al., (44) mentioned that activation of diverse NFκB dimmers might lead to divergent results on neurons, due to the type of cell or the moment of the NFκB activation. In our study we evaluated the expression of NFκB and pNFκB in whole homogenates of hippocampus and frontal lobe, not in individual cells. However, the functional significance of NFκB activation in nervous cell is not fully understood, as NFκB has been reported to have dual role and mediate both survival and death of neurons, depending on the context and probably on its level. Therefore, further studies are needed to explain the role of potential NFκB activation in nervous system, especially under treatment with natural compounds, and its interaction with vital processes in brain (41).
In conclusion, our study demonstrates that quercetin, Hypericum maculatum, Hypericum perforatum and alprazolam exerted anti-inflammatory effects on the several assessed cytokines and reduced the serum corticosterone levels. Thus, decreased lipid peroxidation, either in hippocampus or frontal lobe of the anxious animals, induced by quercetin and the HpE, and at the same time quercetin increased CAT activity in hippocampus, along with enhanced expressions of the NFκB and pNFκB both in hippocampus and frontal lobe. This suggested that natural compounds administration may improve anxiety-like-behavior and offer brain protection by modulation of reactive oxygen species and inflammatory markers. These natural compounds could have another action mechanism, different from that exerted by APZ.
Thus, natural compounds represent an attractive target for the development of new anxiolytic drugs. Moreover, compared to classic benzodiazepines, they show less abuse potential, have less side effects and interference with neuropsychological function. Nevertheless, for both Q and HP toxic effects have also been reported (16). Therefore, a therapeutical concentration of the compounds and their interaction with different other drugs should be carefully established. The significant results obtained for both two species highlight that HM can be used alongside HP as a raw material for obtaining therapeutic products such as formulation of functional foods, or as a food supplement.
Abbreviations: APZ, alprazolam; b.w., body weight; BZD, benzodiazepines; CMC, carboxymethylcellulose; CAT, catalase; CNS, central nervous system; CRF, corticotropin-releasing factor; COX, cyclooxygenase; CK, cytokines; DA, dopamine; FG, FG 7142, N-methyl-9H-pyrido[5,4-b]indole-3-carboxamide; GABA, γ-aminobutyric acid; GPx, gluthation peroxidase; HE, hematoxylin-eosin; HpE, Hypericum extracts; HM, Hypericum maculatum dry extract; HP, Hypericum perforatum dry extract; HPA, hypothalamic-pituitary-adrenal axis; 5-HT, 5-hydroxytryptophan; 5-HIAA, 5-hydroxyindoleacetic acid; IFN-γ, interferon gamma; IFN-c, interferon-c; IL, interleukin; IL-1α, interleukin-1a; i.p., intraperitoneally; LOX, lipoxygenase; MAPK, mitogen activated protein kinase; MCP1, monocyte chemoattractant protein-1; NA, noradrenaline; Nrf2, nuclear factor erythroid 2-related factor 2; NFκB, nuclear transcription factor κB; p38 MAPK, p38 mitogen activated protein kinase; PON2, paraoxonase 2; Q, quercetin; ROS, reactive oxygen species; RANTES, regulated on activation normal T cell expressed and secreted; SOD, superoxide dismutase; SD, standard deviation; TNF-α, tumor necrosis factor alpha; WB, Western blot.
Acknowledgements: This work was financially sustained by the ”Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania (Internal grant no. 4945/23/08.03.2016).
Conflict of interests: None declared.
REFERENCES
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 2011; 164: 1129-1161.
- Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety. Oxid Med Cell Longev 2009; 2: 63-67.
- Masood A, Nadeem AS., Mustafa JM, O’Donnell J. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther 2008; 326: 369-379.
- Schiavone S, Jaquet V, Trabace L, Kraus KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal 2013; 18: 1475-1490.
- Beckhauser TF, Francis-Oliveira J, De Pasquale R. Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. J Exp Neurosci 2016; 10 (S1): 23-48.
- Krolow R, Arcego DM, Noschang C, Weis SN, Dalmaz C. Oxidative imbalance and anxiety disorders. Curr Neuropharmacol 2014; 12: 193-204.
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 2009; 7: 65-74.
- Vogelzangs N, de Jonge P, Smit JS, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry 2016; 3: e249. doi: 10.1038/tp.2013.27
- Costantini D, Marasco V, Moller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates J Comp Physiol B 2011; 181: 447-456.
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009; 1: a001651. doi: 10.1101/cshperspect.a001651
- Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 2015; 8: 77. doi: 10.3389/fnmol.2015.00077
- Lilienbaum A, Israel A. From calcium to NF-kappa B signaling pathways in neurons. Mol Cell Biol 2003; 23: 2680-2698.
- Sevastre-Berghian AC, Fagarasan V, Toma VA, et al. Curcumin reverses the diazepam-induced cognitive impairment by modulation of oxidative stress and ERK 1/2/NF-κB pathway in brain. Oxid Med Cell Longev 2017; 2017: 3037876. doi.org/10.1155/2017/3037876
- Costa LG, Garrick JM, Roque PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016; 2016: 2986796. doi.org/10.1155/2016/2986796
- Oniga I, Toiu A, Benedec D, Tomuta I, Vlase L. Phytochemical analysis of Hypericum maculatum in order to obtain standardized extracts. Farmacia 2016; 64: 171-174.
- Oliveira AI, Pinho C, Sarmento B, Dias AC. Neuroprotective activity of Hypericum perforatum and its major components. Front Plant Sci 2016; 7: 1004. doi: 10.3389/fpls.2016.01004
- Farmacopeea Romana. Bucuresti, Medicala, 1993.
- European Pharmacopoeia. Stresbourg, Council of Europe, 2018.
- Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tiss Organ Cult 2011; 106: 279-288.
- Benedec D, Hanganu D, Oniga I, et al. Achillea schurii flowers: chemical, antioxidant, and antimicrobial investigations. Molecules 2016; 21: E1050. doi:10.3390/molecules21081050
- Hanganu D, Olah NK, Benedec D, et al. Comparative polyphenolic content and antioxidant activities of Genista tinctoria L. and Genistella sagittalis (L.) Gams (Fabaceae) Pak J Pharm Sci 2016; 29: 301-307.
- Trofimiuk E, Walesiuk A, Braszko JJ. St John's wort (Hypericum perforatum) counteracts deleterious effects of the chronic restraint stress on recall in rats. Acta Neurobiol Exp (Wars) 2006; 66: 129-138.
- Mohan L, Rao US, Gopalakrishna HN, Nair V. Evaluation of the anxiolytic activity of NR-ANX-C (a polyherbal formulation) in ethanol withdrawal-induced anxiety behavior in rats. Evid Based Complement Alternat Med 2011; 2011. 327160. doi: 10.1155/2011/327160
- Patki G, Salvi A, Liu H, et al. Tempol treatment reduces anxiety-like behaviors induced by multiple anxiogenic drugs in rats. PLoS One 2015; 10: e0117498. doi: 10.1371/journal.pone.0117498
- Conti M, Morand PC, Levillain P, Lemonnier A. Improved fluorometric determination of malonaldehyde. Clin Chem 1991; 37: 1273-1275.
- Pippenger CE, Browne RW, Armstrong D. Regulatory antioxidant enzymes. Methods Mol Biol 1998; 108: 299-313.
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 1989; 264: 7761-7764.
- Suzen A, Tekin L, Erdemli ME, Erturk N, Aksungur Z, Aktas S. Protective effects of Hypericum perforatum and quercetin in a rat model of ischemia/reperfusion injury of testes. Eur J Pediatr Surg 2018; 28: 96-100.
- Kaminska K, Rogoz Z. The antidepressant- and anxiolytic-like effects following co-treatment with escitalopram and risperidone in rats. J Physiol Pharmacol 2016; 67: 471-480.
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res 2012; 1443: 1-17. doi:10.1016/j.brainres.2012.01.005
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 2009; 34: 1021-1029.
- O'Donovan A, Hughes BM, Slavich GM, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun 2010; 24: 1074-1077.
- Sanchez-Reus MI, Gomez del Rio MA, Iglesias I, Elorza M, Slowing K, Benedi J. Standardized Hypericum perforatum reduces oxidative stress and increases gene expression of antioxidant enzymes on rotenone-exposed rats. Neuropharmacology 2007; 52: 606-616.
- Benedi J, Arroyo R, Romero C, Martin-Aragon S, Villar AM. Antioxidant properties and protective effects of a standardized extract of Hypericum perforatum on hydrogen peroxide-induced oxidative damage in PC12 cells. Life Sci 2004; 75: 1263-1276.
- Maldonado-Bouchard S, Peters K, Woller SA, et al. Inflammation is increased with anxiety- and depression-like signs in a rat model of spinal cord injury. Brain Behav Immun 2016; 51: 176-195.
- Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients 2016; 8: 167. doi: 10.3390/nu8030167
- Nemeth M, Pschernig E, Wallner B, Millesi E. Non-invasive cortisol measurements as indicators of physiological stress responses in guinea pigs. Peer J 2016; 4: e1590. doi: 10.7717/peerj.1590
- Skorzewska A, Wislowska-Stanek A, Lehner M, et al. Corticotropin releasing factor receptor 1 antagonist differentially inhibits freezing behavior and changes gamma-aminobutyric acidergic activity in the amygdala in low- and high-anxiety rats. J Physiol Pharmacol 2017; 68: 35-46 .
- Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol 2009; 621: 46-52.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 2016; 6: 603-621. doi: 10.1002/cphy.c150015
- Bhakar AL, Tannis LL, Zeindler C, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci 2002; 22: 8466-8475.
- Kaltschmidt B, Kaltschmidt C. NF-κB in the nervous system. Cold Spring Harb Perspect Biol 2010; 2: a001271. doi: 10.1101/cshperspect.a001271.
- Sehirli AO, Cetinel S, Ozkan N, et al. St. John's wort may ameliorate 2,4,6-trinitrobenzenesulfonic acid colitis off rats through the induction of pregnane X receptors and/or P-glycoproteins. J Physiol Pharmacol 2015; 66: 203-214.
- Sarnico I, Lanzillotta A, Benarese M, et al. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol 2009; 85: 351-362.
A c c e p t e d : October 30, 2018