Several studies have suggested that ROS can significantly modify platelet functions including platelet surface markers expression, as well as platelet aggregation. It is not clear if superoxide anion scavenging by pre-incubation of platelets ex vivo with SOD can affect platelet aggregation (4). Krotz et al have shown that while platelet-derived superoxide anion did not influence initial aggregation, platelet recruitment to a preformed thrombus following collagen stimulation was significantly attenuated by superoxide dismutase (SOD) (4). NADPH oxidase inhibition in platelets using either a non specific flavin oxidase inhibitor (DPI) or specific oxidase activation inhibitor (apocynin) lead to a similar effect (7).
Moreover, similar effects have been shown by polyphenols which apart from ROS scavenging properties have been shown to inhibit platelet NADPH oxidase in a PKC dependent manner (8). These effects were not only observed using isolated polyphenolic compounds, but also when extracts of certain plants like grape seeds were used (9, 10).
Accordingly, in the present study we aimed to investigate the basal superoxide production from human platelets, along with platelet aggregation induced by collagen and thrombin in relation to cardiovascular risk profile. We also investigated the effects of polyphenol rich extracts of Aronia melanocarpa berries (chokeberry) on platelet superoxide production and platelet aggregation. Chokeberry is a particularly abundant source of polyphenols, which may act as ROS scavengers and could modify the activity of platelet NADPH oxidase. Berries of Aronia melanocarpa along with crowberry show one of the highest contents of phenolic compounds (Gallic Acid Equivalents (GAE) > 20 mg/g), among different natural products studied (11).
The role of superoxide production and studies of the effects of chokeberry extracts on platelets were studied in two distinct groups of subjects – controls without cardiovascular risk factors and patients with significant cardiovascular risk factor profile.
Study populations
We studied platelets isolated from control subjects without risk factors for atherosclerosis (n=15) and 15 subjects with risk factors for atherosclerosis. The clinical characteristics of both groups of patients is presented in Table 2. Subjects were not receiving any medications which could affect platelet function during the 2 weeks preceding the study. The study was approved by Local Bioethics Committee and informed consent was obtained from all individuals.
| Table 1. Risk factor profile of studied subjects. |
 |
| Table 2. Main content of polyphenols within A. melanocarpa extract preparation Aronox. Data based on information supplied by producer (Agropharm SA). |
 |
A. melanocarpa extracts
Extracts of A. melanocarpa used were purchased from Agropharm SA (Poland).
These extracts contain ca. 60% of total polyphenols, including minimum 20% of anthocyanins (Table 2). As total polyphenolic compounds are the major bioactive components of extracts used, in all figures values of concentrations of A. melanocarpa extracts are shown as polyphenol concentrations.
Platelet isolation and aggregation
Citrateted blood (3.2%, 1:9 v/v) was centrifuged at 250 ×g for 20 min in order to obtain platelet rich plasma. Washed platelets were isolated from platelet rich plasma washed twice in PGI2-containing PBS using modified method as described previously suspended (at 2×108 platelet/ml) in calcium-free PBS containing 0.1% albumin.
Platelet aggregation was assessed in using a dual channel Chronolog aggregometer as previously described by us. The baseline value was set using washed platelets while buffer served as full transmittance control. 500µl of washed platelets were equilibrated for 3 minutes at 37°C with continuous stirring and then stimulated with collagen (2 ug/ml) or thrombin (20 mU/ml) to induce aggregation.
Concentrations of collagen and thrombin leading to sub-maximal aggregation were determined in preliminary experiments.
Increasing concentrations of A. melanocarpa extracts were added to platelets and incubated for 2 minutes prior to determination of collagen (2 µg/ml) or thrombin (20mU/ml) induced aggregation. Values were expressed as relative units of maximal aggregation achieved in relation to baseline values obtained as described above.
Platelet superoxide production
Platelet superoxide production was measured using lucigenin enhanced chemiluminescence (LGCL) using a modified version of a method described before (12). Contamination by polymorphonuclear cells of washed platelet preparations was checked microscopically and only samples showing contamination < 1 PMN /108 platelets. Briefly platelets after isolation were equilibrated for 5 minutes in the presence or absence of varying concentrations of A. melanocarpa extracts containing polyphenols. Following this equal number of platelets (105 platelets) were added to a scintillation vial containing 2 ml of 25uM lucigenin solution in Krebs-HEPES buffer. Luminescence was recorded over 25 minutes, or until a plateau was reached using a single channel luminometer (Berthold FB12) modified to maintain constant temperature 37°C as described before. Values were expressed as RLU/sec/105 platelets. Specificity for superoxide was confirmed by pre-incubation with PEG-SOD (250U/ml) or with Tiron (1mM) as described before (13).
Vascular function
Vascular function was measured as flow mediated dilatation in patients with risk factors for atherosclerosis and further related to platelet aggregation studies performed on washed platelets isolated from the same individuals. FMD was studied using Toshiba SSA-340 ultrasound machine using linear 8MHz probe. Patients were rested for at least 15 minutes prior to endothelial function determinations in a dark quiet room. Arm was immobilized using a custom arm rest. Blood pressure cuff was placed on the forearm and brachial artery was located 3-5 cm above the antecubital fossa and baseline brachial artery diameter was measured. Next blood pressure cuff was inflated above the systolic blood pressure value. After 3 minutes flow was restored by releasing the blood pressure cuff and vasorelaxation of brachial artery in response to flow was determined after 2 and 5 minutes. Non endothelium dependent relaxations were determined as vasorelaxations induced by sublingual nitroglycerine administration. FMD values were expressed as % of change in relation to initial diameter.
Statistical analysis
Results are expressed as means ± SEM or medians ± 25th/75th percentiles depending on the distribution of data. n equals to the number of patients. Statistical comparisons between the two groups were made using Students t-test for independent or dependent samples when sample distribution was normal or using non-parametric Mann Whitney U test for samples without normal distribution. Correlations were assessed using Pearson statistics. p values <0.05 were considered statistically significant.
Platelet superoxide production and cardiovascular risk
Basal superoxide production was observed in washed platelets isolated from all studied subjects. Superoxide production was inhibited by SOD (250U/ml) or Tiron (1mM) confirming the specificity of the assays for superoxide. The values of superoxide production varied over 10 fold between different individuals. Moreover, we observed that basal superoxide production was significantly higher in patients with risk factors for atherosclerosis when compared to the control group, free of risk factors (Figure 1A).
 |
Figure 1. Platelet superoxide production (panel A) and aggregation induced by thrombin (20mU/ml; panel B) or collagen (2µg/ml; panel C) in patients with and without cardiovascular risk factors. Superoxide production was measured in washed platelets using lucigenin enhanced chemiluminescence (25µM; n=15). Platelet aggregation was determined as described in methods section. Boxes indicate 75th and 25th percentile. Lines within boxes indicate medians. Lines within boxes indicate medians and whiskers – range of non-outlying values. *p < 0.02 vs. control. |
Platelet aggregation and cardiovascular risk
Variability was also observed between individual subjects in relation to platelet aggregation in response to thrombin (20mU/ml) and collagen (2ug/ml). Concentrations of agents used to stimulate aggregation were determined in preliminary experiments as leading to sub-maximal aggregation. Although a trend was observed towards higher values of platelet aggregation in response to either thrombin or collagen in patients with cardiovascular risk factors, the difference did not reach statistical significance (Figure 1 B and C).
Relationship between platelet superoxide production and platelet function
Previous study has suggested ex vivo that superoxide production by Nox2-dependent NADPH oxidase is important in the regulation of platelet aggregation, we next aimed to study if this effect could be observed in a clinical setting. As both platelet superoxide production and aggregation showed significant variability, we next aimed to determine the relationship between these two parameters in a subgroup of patients. No significant relationship was found between platelet superoxide production and their aggregation in response to collagen (R=-0.1; p=NS) (Figure 2A). Interestingly no significant relationship was observed when it was assessed in subgroups depending on the presence of risk factors for atherosclerosis either. Similarly no significant relationship was found in relation to thrombin induced platelet aggregation (data not shown).
 |
Figure 2. Relationships between platelet aggregation in response to collagen and platelet superoxide production (Panel A; n=12; R=-0.1; p=NS) and between platelet aggregation and endothelial function (Panel B; n=12, R=-0.13; p=NS). |
Relationship between endothelial dysfunction and platelet superoxide production
Endothelial function could be another important determinant of platelet function, particularly in patients with clinical risk factors for atherosclerosis. Accordingly, we have determined a relationship between flow mediated dilatation of brachial artery and collagen induced platelet aggregation. In the studied group of subjects no significant association was found between these parameters (Figure 2B).
Antioxidant effects of Aronia melanocarpa extracts in platelets – relationship to cardiovascular risk factor profile
Next we investigated anti-oxidant properties of the extracts of A. melanocarpa, naturally occurring plant, berries of which are particularly rich in anti-oxidative polyphenols as discussed in the Methods (see Table 2). We observed that polyphenol rich extracts of A. melanocarpa lead to a significant, concentration dependent decrease in superoxide production from washed platelets only in subjects with cardiovascular risk factors, in whom superoxide production was initially increased (Figure 3B), but not in platelets isolated from control group subjects (without cardiovascular risk factors; Figure 3A). Moreover superoxide production in platelets from patients with high cardiovascular risk was decreased by A. melanocarpa extracts to a level comparable to levels observed in a control group (Figure 3A and B). It is also important to note that only A. melanocarpa extracts were effective in anti-oxidative action in human platelets when concentrations of polypohenols reached levels of 1µg/ml, at which concentrations, polyphenols of A. melanocarpa may exert free radical scavenging effect rather than inhibitory effect toward platelet oxidases (N. Ryszawa, T Guzik, unpublished data).
 |
Figure 3. Effects of increasing concentrations of polyphenols from A. melanocarpa berry extracts on superoxide production from washed platelets isolated from control subjects (panel A) and patients with risk factors for atherosclerosis (panel B). Superoxide production was determined using LGCL (20µM) as described above. Concentrations shown on X axis refer to total polyphenol content within A. melanocarpa extract solution. Data in panel A are presented as medians (lines within boxes) and 75th and 15th percentile (boxes) (distribution not normal). Data in panel B are shown as means +/-SEM. (normal distribution) * - p<0.05 vs. native using appropriate tests depending on data distribution. |
Effects of Aronia melanocarpa extracts on platelet function.
Next the effects of A. melanocarpa extracts were studied in relation to platelet aggregation in both control subjects and patients with significant cardiovascular risk factors. In contrary to the effects on superoxide production, A. melanocarpa extracts caused significant inhibition of platelet aggregation induced by thrombin or by collagen in both studied groups of subjects i.e. in both control group and patients with significant cardiovascular risk (Figure 4). Importantly, no difference in dose range that caused inhibition of aggregation was observed between the groups and A. melanocarpa polyphenol rich extracts exerted their protective action at relatively high concentrations. At lower concentrations (0.001-1 µg of polyphenols per ml) no significant effect on platelet aggregation was observed (data not shown).
 |
| Figure 4. Effects of increasing concentrations of polyphenols from A. melanocarpa berry extracts on platelet aggregation induced by thrombin (20mU/ml; top panels) and collagen (2ug/ml; bottom panels) from control subjects (right panels) and patients with risk factors for atherosclerosis (left panels). Boxes indicate 75th and 25th percentile. Lines within boxes indicate medians and whiskers – range of non-outlying values. *p < 0.02 vs. control. |
Potential role of platelet derived NO in anti-aggregatory effects of A. melanocarpa extracts
As the anti-aggregatory effects of A. melanocarpa extracts appeared to occur independently of it’s effects on superoxide production, we next investigated a possibility that major anti-aggregatory effect of relatively high concentrations of A. melanocarpa extracts are mediated by it’s effects on NO metabolism. NO has been shown to be released from platelets and cNOS is one of the important targets of polyphenolic compounds actions.
Accordingly platelet aggregation was studied in washed platelets isolated from subjects without and with cardiovascular risk profile in the presence and in the absence of NOS inhibitor L-NAME (200µM). We observed that pre-incubation of platelets with L-NAME did not change platelet aggregation at baseline induced by either thrombin or collagen (Figure 5). Moreover, we observed that L-NAME did not modify anti-aggregatory properties of A. melanocarpa extracts, indicating that NOS derived NO from platelets is not involved in protective, direct anti-aggregatory effects of A. melanocarpa on platelets (Figure 5).
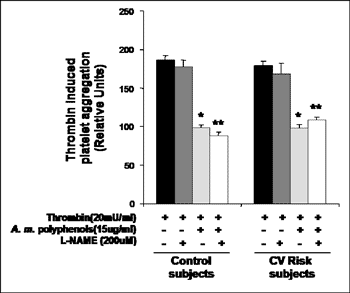 |
Figure 5. Effects of A. melanocarpa polyphenols on platelet aggregation are independent of platelet NOS. Washed platelets were pre-incubated with 200µM L-NAME prior to the incubation with A. melanocarpa extracts (15µg/ml) and determination of thrombin (20mU/ml) dependent platelet aggregation. Parallel experiments were performed in platelets isolated from control individuals and from subjects with cardiovascular risk factors. *-p<0.02 vs native; *-p<0.02 vs native+L-NAME |
Oxidative stress plays an important role in the regulation of cellular function in cardiovascular disease1. This has been shown in blood vessels and vascular cells in numerous studies (14). Much less attention has been devoted to the characterization and understanding of the mechanisms of oxidative stress in human platelets and their function.
In the present study, we were able to successfully measure basal (as opposed to induced in vitro by artificial agonists) superoxide production from platelets. We show that it is significantly increased in patients with cardiovascular risk profile when compared to controls, while platelet aggregation in response to either collagen or thrombin is only borderline higher in this group of subjects. Interestingly, no relationship was found between platelet aggregation ex vivo and platelet superoxide production in either of studied groups. No correlation was found between endothelial function (measured by FMD) and platelet aggregation ex vivo either. Polyphenol rich extracts of A. melanocarpa berries caused significant concentration dependent decrease in superoxide production only in patients with cardiovascular risk factors, while no effect was observed in the control group. A. melanocarpa extracts abolished the difference in superoxide production between risk factor patients and controls. A. melanocarpa extracts exerted significant concentration dependent anti-aggregatory effects in both studied groups, which indicated that this effects may be independent of it’s ability to modulate superoxide production. These effects were similar irrespective of aggregation inducing agent (collagen or thrombin). Moreover, anti-aggregatory effects of A. melanocarpa extracts appear to be independent of platelet NO release as NOS inhibition by L-NAME did not lead to their abrogation.
Ability of platelets to produce superoxide anion has been demonstrated before, however most studies used stimuli which have been shown to stimulate oxidative burst in neutrophils e.g. PMA (6). Basal superoxide production in platelets was not studied to such an extent so far, mainly because studies used often healthy subjects, who (also in a present study) demonstrate very low levels of basal superoxide production. We show here that already at baseline conditions, platelets from cardiovascular risk patients produce ca. 10 times more superoxide than controls.
It is also noteworthy that we used low (25µM) concentration of lucigenin in order to diminish risk of lucigenin redox cycling which has been a problem with higher lucigenin concentrations (12). It is also important that we performed assays at 37°C, which greatly increases sensitivity of superoxide assays in living cells (12). The increase in baseline superoxide production described in the present paper may have several important functional consequences.
Superoxide dismutase (SOD), as well as NADPH oxidase inhibitors (DPI, apocynin), inhibit platelet recruitment to a preformed thrombus following collagen stimulation (4). ADP in supernatants of collagen-activated platelets was decreased in the presence of SOD, resulting in lower ADP concentrations available for recruitment of further platelets (4). Interestingly while a vast number of papers have looked at the effects of SOD on different aspects of platelet activation, no solid data is available on the effects SOD would have on agonist induced platelet aggregation. Krotz et al did not find evidence that platelet-derived superoxide would influence agonist induced platelet aggregation without pre-formed thrombus (4).
Superoxide release by both platelet and the endothelial cell is a key factor in regulating platelet-endothelial cell interaction, a primary event in platelet aggregation (15).
Finally, platelet NADPH oxidase dependent superoxide production is important in regulating platelet CD40 ligand expression, as patients with gp91phox deficiency showed greatly abolished CD40 ligand induction by several stimuli (16), which indicates potential importance of platelet NADPH oxidase and superoxide production in the clinical setting.
Conflicting results have been obtained when the effects of exogenously delivered ROS on in vitro platelet aggregation were studied. In some studies xanthine-xanthine oxidase system caused decrease, rather than an increase of aggregation (17).
The relationship between platelet superoxide production and cardiovascular risk factors found here is in agreement with previous studies that show that agonist stimulated platelet superoxide production is higher in patients with cardiovascular risk factors like hypertension (18) or diabetes (6). Similarly platelet superoxide production is increased in other diseases usually associated with increased vascular oxidative stress like nitrate tolerance18. Angiotensin II may play an important role in stimulating platelet superoxide production through activation of NAD(P)H oxidase via the AT1 receptor and PKC.
The findings of the present study are in line with data previously published in regard to platelet oxidative stress and indicate that regulation of oxidative stress in platelets by risk factors may be similar to human vasculature in which superoxide production is directly related to number of risk factors (19).
The major sources of superoxide production in platelets were studied so far only in relation to agonist stimulated superoxide production, rather than basal, and show that apart from NADPH oxidases, dysfunctional platelet cNOS (NOS III) may be an important source of superoxide in hypertension or diabetes (6). We have not addressed this issue in the present study. We have, in turn, investigated the relationship between platelet superoxide production and platelet aggregation induced by collagen or thrombin. There was no significant relationship between those parameters in neither patients with cardiovascular risk, nor in subjects from the control group. None of the previous studies looked at this aspect of platelet oxidative stress.
Lack of such association, does not however indicate that platelet superoxide production is not important in regulating platelet aggregation. It is possible that presence of risk factors and endothelial dysfunction is not sufficient to significantly change aggregation but may be enough to increase superoxide production in platelets. Therefore, oxidative stress in platelets may precede the development of increased aggregation. This potential explanation is in line with our observation that while cardiovascular risk factors greatly increase platelet superoxide production, platelet aggregation in response to collagen or thrombin remained not significantly changed. It is possible that change of aggregation occurs at more exaggerated stages of cardiovascular diseases, and in those patients, such as unstable angina patients, the relationship between aggregation and platelet oxidative stress may become more evident. It is also important to point out that in vivo the interactions and role and bioavailability of studied agonists like thrombin (20) or collagen may be different to conditions of our in vitro aggregation studies.
Considering the importance of free radicals in modulating platelet function several studies investigated the effects of various anti-oxidants on human platelets. These mainly included polyphenol-rich natural compounds including grape seeds, pomegranate juice or red wine components, particularly resveratrol (9, 21, 22). Bioactive substances found in numerous foods (23,24), can be successfully and safely used to modify various cellular functions including oxidative stress. Particularly plant derived extracts create a good opportunity for development of novel treatment strategies (25, 26).
In the present study we have, for the first time, investigated the effects of extracts from chokeberry (Aronia melanocarpa) on platelet superoxide production and agonist induced aggregation in vitro. Berries of A. melanocarpa belong to the most abundant sources of polyphenols mainly anthocyanins and are widely available in the form of either juice, berries themselves or extracts particularly enriched in polyphenols and anthocyanins. One of the initial studies that compared the content of phenolic compounds in different natural products has shown that berries of aronia along with crowberry (11). The content of total phenolics in the extracts determined spectrometrically according to the Folin-Ciocalteu procedure and calculated as gallic acid equivalents (GAE) indicated that GAE of aronia berries exceeded 20 mg/g, while majority of natural well known sources of ployphenols showed values ca. 10-12 mg/g (11).
There are several studies indicating potential beneficial effects of A. melanocarpa berry extracts. These include anti-cancer effects, mediated primarily by increase of tumor suppressor genes as well as by reduction of oxidative stress and resulting DNA damage important for the proliferation of cancer cells (27, 28, 29). Interestingly A. melanocarpa berry extracts have been also implicated in the treatment of several other conditions including oligospermia (30). Chokeberry extracts show significant protective effects in the cardiovascular system, and initial clinical studies have confirmed their usefulness (31). In their study Kowalczyk et al have shown that anthocyanins from chokeberry decrease lipid peroxidation which may be potentially used to combat oxidative stress in cardiovascular risk subjects, which may make them potentially interesting drugs for adjuvant cardiovascular therapy (31), and could be useful also in other conditions related to vascular function changes (32). Our study extends those findings by showing important anti-platelet effects of A. melanocarpa berry extracts, particularly in patients with significant cardiovascular risk factors. The mechanisms of those effects remain however unclear. They may be partially mediated by anti-oxidant effects of the extracts. The mechanism of actions of polyphenols on platelets are mediated primarily through their free radical scavenging effects but they have also been shown to inhibit NADPH oxidases and PKC which regulates them (8). Finally polyphenols have been suggested to increase endogenous anti-oxidant capacity through enhancement of SOD activity, which may be important in the regulation not only of platelet aggregation, but maybe more importantly of vascular superoxide production (33).
It is however important to note, that even in platelets from healthy control subjects in which antioxidant effects of A. melanocarpa extracts are minor, their anti-aggregatory capacity remains similar to observed in platelets from subjects with cardiovascular risk factors (in whom antioxidant effects are pronounced). The latter indicates some other additional potential mechanism additionally involved in the inhibition of platelet aggregation by chokeberry extracts. As nitric oxide exerts numerous protective aniti-aggregatory effects, and that it can actually be produced within platelet, it is possible that polyphenol rich extracts of A. melanocarpa could inhibit platelet aggregation at least in part by increasing platelet NOS activity. Indeed isolated polyphenols have been shown to have an ability to stimulate NO production and NO donors significantly inhibit aggregation (34). However, experiments presented here do not confirm the hypothesis that anti-aggregatory effects of chokeberry extracts are related to NOS activation within the platelet. Further studies are warranted to determine exact mechanisms of anti-aggregatory effects of studied A. melanocarpa berry extracts.
In conclusion, we find that superoxide production is increased in platelets obtained from patients with cardiovascular risk factors even in the absence of major abnormalities of platelet aggregation in vitro. Polyphenol rich extracts of A. melanocarpa berries show very significant anti-oxidant and anti-aggregatory effects in human platelets, particularly in patients with cardiovascular risk factors. Further clinical studies are warranted to confirm present findings in an in vivo situation in humans.
Acknowledgments: This work was supported by Polish Ministry of Education and Science (grant no. 2PO5A 01227). We are grateful to Mrs Jolanta Reyman for her excellent technical help and expertise in platelet aggregation studies.
- Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today. 2006; 11: 524-533.
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54: 469-487.
- Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol. 2002; 53: 515-524.
- Krotz F, Sohn HY, Gloe T, et al. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 2002; 100: 917-924.
- Sudic D, Razmara M, Forslund M, Ji Q, Hjemdahl P, Li N. High glucose levels enhance platelet activation: involvement of multiple mechanisms. Br J Haematol 2006; 133: 315-322.
- Dixon LJ, Hughes SM, Rooney K, et al. Increased superoxide production in hypertensive patients with diabetes mellitus: role of nitric oxide synthase. Am J Hypertens 2005;18: 839-843.
- Begonja AJ, Teichmann L, Geiger J, Gambaryan S, Walter U. Platelet regulation by NO/cGMP signaling and NAD(P)H oxidase-generated ROS. Blood Cells Mol Dis 2006; 36: 166-170.
- Pignatelli P, Di Santo S, Buchetti B, Sanguigni V, Brunelli A, Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. Faseb J 2006; 20: 1082-1089.
- Vitseva O, Varghese S, Chakrabarti S, Folts JD, Freedman JE. Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. J Cardiovasc Pharmacol 2005; 46: 445-451.
- Olas B, Wachowicz B, Bald E, Glowacki R. The protective effects of resveratrol against changes in blood platelet thiols induced by platinum compounds. J Physiol Pharmacol 2004; 55: 467-476.
- Kahkonen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem 1999; 47: 3954-3962.
- Guzik TJ, Channon KM. Measurement of vascular reactive oxygen species production by chemiluminescence. Methods Mol Med 2005;108:73-89.
- Guzik TJ, Sadowski J, Guzik B, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 2006; 26: 333-339.
- Guzik TJ, Sadowski J, Kapelak B, et al. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol 2004; 24:1614-1620.
- Cerwinka WH, Cooper D, Krieglstein CF, Ross CR, McCord JM, Granger DN. Superoxide mediates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am J Physiol Heart Circ Physiol 2003; 284: H535-H541.
- Pignatelli P, Sanguigni V, Lenti L, et al. gp91phox-dependent expression of platelet CD40 ligand. Circulation 2004; 110: 1326-1329.
- Ambrosio G, Golino P, Pascucci I, et al. Modulation of platelet function by reactive oxygen metabolites. Am J Physiol 1994; 267: H308-H318.
- McVeigh GE, Hamilton P, Wilson M, et al. Platelet nitric oxide and superoxide release during the development of nitrate tolerance: effect of supplemental ascorbate. Circulation 2002; 106: 208-213.
- Guzik TJ, West NE, Black E, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res 2000; 86: E85-E90.
- Pajdak W, Radwan J, Guzik TJ. Cleavage of prothrombin bound in immune complexes results in high thrombin enzymatic activity. J Physiol Pharmacol 2004; 55: 477-484.
- Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 2000; 71: 1062-1076.
- Shigematsu S, Ishida S, Hara M, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med 2003; 34: 810-817.
- Dulak J. Nutraceuticals as anti-angiogenic agents: hopes and reality. J Physiol Pharmacol 2005; 56 Suppl 1: 51-69.
- Heinrich M, Leonti M, Nebel S, Peschel W. “Local Food - Nutraceuticals”: An example of a multidisciplinary research project on local knowledge. J Physiol Pharmacol 2005; 56 Suppl 1: 5-22.
- Schaffer S, Schmitt-Schillig S, Muller WE, Eckert GP. Antioxidant properties of Mediterranean food plant extracts: geographical differences. J Physiol Pharmacol 2005; 56 Suppl 1: 115-124.
- Kapiszewska M, Soltys E, Visioli F, Cierniak A, Zajac G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J Physiol Pharmacol 2005; 56 Suppl 1: 183-197.
- Lala G, Malik M, Zhao C, et al. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer 2006; 54: 84-93.
- Bermudez-Soto MJ, Larrosa M, Garcia-Cantalejo JM, Espin JC, Tomas-Barberan FA, Garcia-Conesa MT. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J Nutr Biochem 2006.
- Malik M, Zhao C, Schoene N, Guisti MM, Moyer MP, Magnuson BA. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr Cancer 2003; 46: 186-196.
- Pawlowicz P, Stachowiak G, Bielak A, Wilczynski J. [Administration of natural anthocyanins derived from chokeberry (aronia melanocarpa) extract in the treatment of oligospermia in males with enhanced autoantibodies to oxidized low density lipoproteins (oLAB). The impact on fructose levels]. Ginekol Pol 2001; 72: 983-988.
- Kowalczyk E, Kopff A, Niedworok J, Kopff M, Jankowski A. Anthocyanins—an adjunct to cardiovascular therapy? Kardiol Pol 2002; 57: 332-336.
- Mussa S, Guzik TJ, Black E, Dipp MA, Channon KM, Taggart DP. Comparative efficacies and durations of action of phenoxybenzamine, verapamil/nitroglycerin solution, and papaverine as topical antispasmodics for radial artery coronary bypass grafting. J Thorac Cardiovasc Surg 2003; 126: 1798-1805.
- Guzik TJ, Olszanecki R, Sadowski J, et al. Superoxide dismutase activity and expression in human venous and arterial bypass graft vessels. J Physiol Pharmacol 2005; 56: 313-323.
- Homer KL, Wanstall JC. Inhibition of rat platelet aggregation by the diazeniumdiolate nitric oxide donor MAHMA NONOate. Br J Pharmacol 2002; 137: 1071-1081.