SERUM NEUTROPHIL GELATINASE-ASSOCIATED LIPOCALIN (NGAL) CORRELATES WITH CLINICAL AND ENDOSCOPIC ACTIVITY IN ULCERATIVE COLITIS BUT FAILS TO PREDICT ACTIVITY IN CROHN’S DISEASE
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are lifelong inflammatory bowel diseases (IBD) characterized by the periods of exacerbations and remissions. Their importance is growing due to increase of incidence and prevalence in developed countries (1). At present, the goal of treatment is to achieve and maintain deep clinical and endoscopic remission, reflected by mucosal healing that can be reached in up to 80% of patients through development of individualized therapy (2). Currently, the gold standard for assessing the extent and severity of mucosal injury remains colonoscopy with biopsy, however, this method is not apt for frequent repetitions (3). For this reason, simple laboratory indicators in blood, stool and even in saliva capable to monitor disease activity under treatment and predict the relapses are being intensively sought (3-6).
A new promising marker of IBD activity is neutrophil gelatinase-associated lipocalin (NGAL), (known also as lipocalin-2) measured in serum or feces. NGAL is an acute phase glycoprotein covalently bound to matrix metalloproteinase-9 (7, 8). Under physiological conditions it is secreted in small amounts by neutrophils, macrophages, epithelial cells, smooth muscle cells, hepatocytes, adipocytes and neurons (9). Increased NGAL concentrations in serum associates the injury to epithelial cells of the gastrointestinal tract, respiratory tract or renal tubules (8-10). A number of studies have evidenced the role of NGAL as a diagnostic and prognostic biomarker for acute or chronic kidney disease, sepsis and acute pancreatitis as well as gastric, colorectal, pancreatic and biliary cancer (7, 13-18).
Although the biological role of NGAL is not fully understood, it is known that it exerts a bacteriostatic effect by hindering access of bacteria to the iron. By decreasing cellular uptake of the iron NGAL also modulates cell proliferation, apoptosis and differentiation (7, 19, 20).
Increased concentration of urinary MMP9/NGAL complex and NGAL in stool has been reported in IBD patients (20, 22). In two studies serum NGAL concentrations were significantly higher in UC and CD patient than in healthy individuals or patients with irritable bowel syndrome (23, 24), but relationships between serum levels of NGAL and clinical or endoscopic determinants of IBD activity remain unclear (21-27). The present study was designed to evaluate the utility of serum NGAL as a non-invasive marker of clinical and endoscopic severity of UC and CD.
MATERIAL AND METHODS
Study design
The study was performed prospectively from January 2015 to October 2016 among patients with IBD and normal kidney function (eGFR > 90 ml/min) admitted to our tertiary referral gastroenterological department. The study was approved by Ethics Committee of the Medical University of Silesia and informed consent was received from each patient.
A total of 120 patients (68 women, 52 men) with CD (n = 79) and UC (n = 41) were included into the study. The blood and stool samples were collected from each patient, and the colonoscopy was performed on the consecutive day. The evaluation of clinical activity of the disease was done on the day of blood and stool collection, before preparation to colonoscopy. Endoscopic activity in patients with UC was classified as remission/mild (0 – 1 points) or moderate /severe (2 – 3 points) according to the Mayo endoscopic score. A Simple Endoscopic Score for Crohn’s disease (SES-CD) was used to describe CD endoscopic activity, where inactive/mild was defined by 3 – 7 points and moderate / severe by over 7 points (26). Additional imaging methods such as magnetic resonance or computer tomography enterography were applied to assess disease activity in some CD patients. Clinical disease activity in UC was determined by total Mayo score, where the remission/mild disease was diagnosed with 0 – 4 points and moderate / severe with 5 – 12 points (27). In patients with CD the clinical activity was defined by the CDAI and was recognized as inactive /mild disease for ≤ 220 points and moderate/severe for > 220 points (28). IBD patients were considered to have inactive disease if total Mayo score was 0 – 1 and CDAI was < 150.
Serum and fecal samples
Blood morphology including white blood cells and platelet count, hemoglobin level, iron level, ESR and serum biochemistry, including creatinine, γ-glutamyltranspeptidase (GGTP), alanine transaminase (ALT) and C-reactive protein (CRP) were determined by flow cytometry (Sysmex XT-1800i) and colorimetry (Olympus AU680).
Five ml of venous blood was obtained, centrifuged for 15 min at 1500g and immediately stored in small aliquots at –80ºC. Serum levels of NGAL were measured using a commercially available sandwich-type enzyme-linked immunosorbent assay kit (BioVendor R&D Systems, Czech Republic) according to manufacturer’s protocol. NGAL levels were calculated from the standard calibration curve.
The stool samples were prepared according to the manufacturer’s instruction (PhiCal® Calprotectin ELISA Kit, Bensheim, Germany) using the fecal extraction device. The small amounts of stool were suspended in 0.75 ml of extraction buffer. After shaking and sedimentation, the supernatants were stored in small aliquots at –80ºC for later analysis. Calprotectin was expressed in micrograms per gram of feces (µg/g).
Statistical analysis
Statistical analysis was performed using Statistica 13.1 software (StatSoft, Polska). The age and BMI were normally distributed and Student’s t-test was used for analysis. These data were presented as mean ± S.D. For laboratory data not showing normal distribution the nonparametric tests were used; the Mann-Whitney U test for analysis of quantitative variables and the Kruskall-Wallis analysis of variance for comparisons between more than two groups. These data were presented as median (interquartile range) values. The Pearson Chi-squared test was used for comparisons of independent groups. A P value of less than 0.05 was considered significant. Receiver operating characteristic (ROC) analyses for serum NGAL, CRP, ESR and fecal calprotectin levels were performed and the area under the curve (AUC-ROC) was obtained to compare the significance of these variables for the differentiation between inactive and active IBD. The optimal cut-off points with respective sensitivity and specificity values were estimated from the ROC curve analysis. The correlation analyses between serum NGAL and other laboratory parameters were performed using the Spearman’s rank test. Associations of investigated variables with IBD activity was examined by multivariate logistic regression analysis, to which only variables significant in univariate analysis were included.
RESULTS
Patient characteristics
Generally, the UC and CD groups were similar in terms of demographic and clinical data except that the body mass index (BMI) was significantly lower in CD patients and the incidence of arthropathy and surgical history were significantly higher in CD group (Table 1). There was also no significant difference in laboratory results between UC and CD patients, including NGAL levels: 62.7 ng/ml (45.6 – 90.8) versus 60.7 (43.9 – 85.2) (P = 0.26), respectively. NGAL levels were similar in IBD patients of both genders: females 59.4 ng/ml (45.2 – 77.4) and males 62.3 ng/ml (42.9 – 91.5) (P = 0.44). Ex-smokers had significantly higher NGAL levels than those who never smoked: 60.2 ng/ml (46.6 – 104.7) versus 53.2 ng/ml (40.7 – 81.7) (P = 0.04), although the difference between those that currently smoke and those who never smoked did not reach statistical significance (65.6 ng/ml (54.2 – 90.8) versus 53.2 ng/ml (40.7 – 81.7), P = 0.09). Used medications did not show significant influence on NGAL levels; in 5-ASA users it was 62.6 (44.2 – 95.5) ng/ml, in corticosteroid users 63.1 (45.2 – 90.8) ng/ml, in patients under immunomodulation drugs 63.9 (45.6 – 92.3) ng/ml and in anti-TNF-α users 61.9 (44.2 – 87.4) ng/ml. NGAL levels were significantly higher in patients with active 64.1 (46.3 – 90.9) ng/ml than inactive IBD 53.6 (38.5 – 74.9) ng/ml (P = 0.05) (Fig. 1). Significant differences between active and inactive IBD were also detected in hemoglobin concentrations: 12.2 g/dl (10.6 – 13.1) versus 12.9 g/dl (12.1 – 14.1) (P = 0.001), platelets counts: 358 G/l (284 – 449) versus 282 G/l (236 – 329) (P = 0.001), ESR: 24 mm/h (11 – 37) versus 8.5 mm/h (4.5 – 15.0) (P = 0.0001), CRP levels: 13.0 mg/l (3.9 – 34.4) versus 4.1 mg/l (1.7 – 7.5) (P = 0.001) and iron levels: 42.0 mg/l (22.0 – 74.0) versus 67.0 mg/l (40.5 – 113) (P = 0.006). In multivariate analysis the only variable significantly associated with disease activity was ESR: OR 1.1 (95% CI 1.01 – 1.13, P = 0.01).
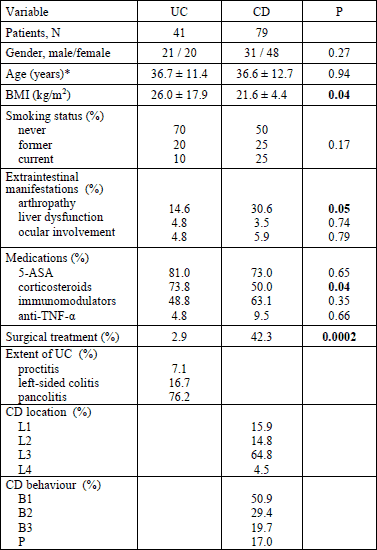
 |
Fig. 1.NGAL in active and inactive IBD patients. Box plots indicate the median, the 25th and 75th percentile. Error bars indicate maximum and minimum. CRP, C reactive protein; NGAL, neutrophil gelatinase-associated lipocalin |
Serum neutrophil gelatinase-associated lipocalin in ulcerative colitis
Serum NGAL level significantly rose with increasing clinical and endoscopic activity assessed by Mayo score (Table 2). On clinical ground the NGAL levels significantly differentiated patients with active disease (64.2 ng/ml (46.9 – 93.5)) from patients in complete remission (38.3 ng/ml (31.5 – 42.4), P = 0.005) (Fig. 1). This goal was also achieved with fecal calprotectin concentration: 318 µg/g (128 – 537) versus 10.2 µg/g (4.9 – 51.3) (P = 0.005), ESR: 15.5 mm/h (8.0 – 44.0) versus 2.0 mm/h (1.0 – 8.0) (P = 0.02) and iron concentration: 33.5 µg /l (22.5 – 74.5) versus 153 µg/l (91.5 – 222.0) (P = 0.01).

The serum NGAL levels were significantly higher in patients with endoscopically active (Mayo score 2 – 3) than inactive disease (Mayo score 0 – 1) (Fig. 1). Patients with complete mucosal healing (only 4 patients) had insignificantly lower NGAL concentrations than patients with active inflammation assessed by histology: 38.8 ng/ml (31.5 – 68.2) versus 63.8 ng/ml (46.9 – 90.9) (P = 0.08).
Serum NGAL level was not related to the extent of the disease being 42.8 ng/ml (41.9 – 83.6) in isolated proctitis, 54.4 ng/ml (45.1 – 79.8) in left-side colitis and 63.2 ng/ml (45.8 – 93.5) in pancolitis (P = 0.51). Type of treatment also did not impact NGAL level.
Serum NGAL level correlated negatively with iron and hemoglobin levels and positively with CRP, ESR and Mayo score (Table 3). There was no single variable significantly associated with the active UC in multivariate analysis.

Serum neutrophil gelatinase-associated lipocalin in Crohn’s disease
The levels of NGAL did not differ between various grades of disease activity determined by CDAI or endoscopic activity expressed as SES-CD (Table 4). Only an insignificant trend toward higher NGAL levels in endoscopically active (according to SES-CD) as compare to inactive CD was detected (72.5 (51.1 – 97.7 versus 56.9 (42.1 – 73.3) ng/ml; P = 0.06). There was no significant difference in serum NGAL level depending on CD location (colon 62.7 (48.0 – 96.7) ng/ml versus small intestine 36.5 (26.5 – 64.0 ng/ml, P = 0.07) or disease predominant form (inflammatory 65.6 (50.1 – 90.8) ng/ml, stricturing 47.8 (42.1 – 88.5) ng/ml and penetrating 60.7 (36.5 – 69.6) ng/ml; P = 0.33). Weak correlations between serum NGAL and CRP (r = 0.380), platelet count (r = 0.237), iron (r = 0.2366) and fecal calprotectin (0.249) were found in CD.
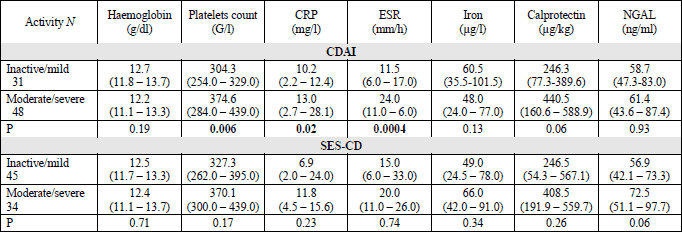
Diagnostic accuracy of neutrophil gelatinase-associated lipocalin in distinguishing active from inactive inflammatory bowel disease
Receiver operating curve (ROC) analyses with calculations of area under the curve (AUC) were performed to evaluate the accuracy of NGAL in discriminating active from inactive IBD. A cut-off point of 42.1 ng/ml was optimal to distinguish active from inactive IBD with a sensitivity of 86% and a specificity of 68% (AUC 0.61, 95%; CI 0.51 – 0.72). In analysis restricted to UC patients the optimal cut-off to discriminate active and inactive form was 43.6 ng/ml with AUC 0.79 (95% CI; 0.65 – 0.93), sensitivity of 96% and a specificity of 50%. There were no significant differences between the AUCs of NGAL and CRP: 0.83 (95% CI 0.69 – 0.96) or ESR: 0.88 (95% CI 0.76 – 1.0). The ROC analysis combining 3 variables - NGAL, CRP and ESR gave the AUC of 0.94. In UC patients for discriminating endoscopically active from inactive inflammation the NGAL, fecal calprotectin and CRP gave the AUCs of 0.758, 0.571 and 0.699, respectively (differences not significant) (Fig. 2). The cut-off value of 43.6 ng/ml recognized patients with endoscopic remission with sensitivity of 96%, specificity of 54%, positive predictive value of 77% and negative predictive value of 80%.
 |
Fig. 2.Comparison of ROC curves showing ability of NGAL, CRP and calprotectin to distinguish patients with endoscopically active from patients with endoscopic remission in ulcerative colitis. CRP, C reactive protein; NGAL, neutrophil gelatinase-associated lipocalin. |
For distinguishing CD patients with active from patients with inactive endoscopic images of CD the AUC was 0.608 for NGAL, 0.692 for CRP and 0.683 for fecal calprotectin (differences not significant) (Fig. 3). For this purpose the optimal cut-off value of NGAL was 72.5 ng/ml (sensitivity 48%, specificity 83%, positive predictive value 86%, negative predictive value 43%).
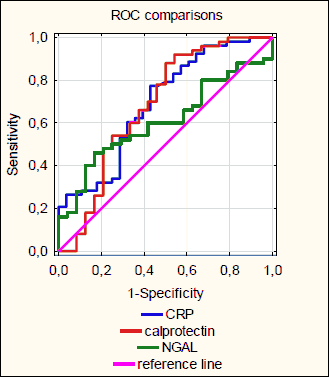 |
Fig. 3.Comparison of ROC curves showing ability of NGAL, CRP and calprotectin to distinguish patients with endoscopically active from patients with inactive Crohn’s Disease. CRP, C reactive protein; NGAL, neutrophil gelatinase-associated lipocalin. |
DISCUSSION
The main finding of the present study was that serum NGAL levels paralleled increasing clinical activity of UC evaluated by Mayo score and it also discriminated different stages of endoscopic severity of this disease. By contrast, only insignificant association of NGAL with endoscopic activity of CD was found with no relationships with clinical activity scores. This finding suggests that serum NGAL mostly derives from inflamed mucosa and probably intramural inflammation has insignificant influence on its synthesis. Lack of correlation between NGAL and white blood cell count and lack of influence of corticosteroids (increasing the amount of neutrophils) on NGAL levels suggest that neutrophils are not a major source of NGAL in IBD patients.
In previous studies NGAL level in IBD patients was significantly higher than in control populations consisting of persons with healthy bowel (9, 23, 24), but NGAL probably cannot discriminate between UC and CD patients. In the present study and the study by Oikonomou et al. (23) the serum NGAL levels did not differ in UC and CD patients, but Yesil et al. found higher concentration of NGAL in UC patients (24). These conflicting results may come from different enrollments of CD patients with extensive colonic involvement, in which NGAL level is believed to be higher than in the disease restricted to the ileum. Moreover, NGAL level may depend on the area of the injured mucosa. Yesil et al. found significant differences in serum NGAL level with respect to extension of UC and CD (24). In our study NGAL concentration in UC patients showed increasing tendency across the whole spectrum of large bowel involvement, but the differences were not significant, probably due to small number of patients.
In our study NGAL level correlated significantly with CRP and ESR in UC patients, however, these correlations in CD patients were either weak or absent. We also found significant negative correlation between NGAL and iron concentration both in UC and CD. This could result from more severe forms of IBD characterized by prolonged loss of blood, but also might reflect a role of NGAL in iron sequestration (19).
Literature data on relationships between NGAL and clinical scoring systems of IBD are inconclusive. Commonly used indices such as CCAI, Mayo score or CDAI were criticized for highly subjective measures. Therefore, we decided to compare NGAL levels with more objective endoscopic features of inflammatory activity. In UC patients NGAL levels perfectly reflected endoscopic Mayo score, discriminating inactive from mild and severe stage of disease. The performance of NGAL in discrimination between active and inactive UC estimated from AUC-ROC was 0.758, which was slightly better than this for CRP and fecal calprotectin. Setting the NGAL cut-off level at 43.6 ng/ml diagnosed endoscopic remission with sensitivity of 96% and specificity of 54%. Similar results were reported by Thorsvik et al. for fecal NGAL, which correlated closely with Mayo endoscopic score and fecal calprotectin concentration (25). In this Norwegian study plasma NGAL performed worse than fecal NGAL for both UC and CD.
The drugs used in IBD treatment variably diminish inflammation and subsequently may modify NGAL level (23, 31). It has been reported that serum NGAL was significantly lower in UC patients on 5-ASA alone in comparison with those treated with other drugs, probably because of more quiescent disease in the former group (23, 24). Recently, it has been shown that NGAL level was reduced by infliximab (IFX) only in responders to this therapy, and consequently was suggested a new surrogate marker for the assessment of mucosal healing in UC patients treated with IFX (26). In our study the type of treatment had no significant impact on NGAL levels.
The major limitation of our study is a relatively low number of patients. Furthermore, the colonoscopies were not video-recorded, hence primary classification could not be re-assessed.
In conclusion, serum NGAL is a valuable biomarker of inflammatory activity in UC patients, complementary to commonly used laboratory tests. In this study we confirmed that NGAL can discriminate patients with endoscopic and clinical remission especially in UC. Taking into account conflicting results from different reports, the diagnostic efficacy of NGAL, especially in context of non-invasive monitoring of therapy effects needs to be confirmed in larger studies.
Authors’ contributions: All authors have made substantial contribution to this work. A. Budzynska, M. Gawron-Kiszka, E. Nowakowska-Dulawa and M. Hartleb prepared the concept and design of the study. A. Budzynska, E. Nowakowska-Dulawa and M. Hartleb raised funds and E. Nowakowska-Dulawa and M Gawron-Kiszka got approval from the Ethics Committee. A. Budzynska, M. Gawron-Kiszka, J. Spiewak, M. Lesinska, M. Waluga, M. Kukla worked on acquisition of data. A. Budzynska made analysis and interpretation of data. Budzynska and Gawron-Kiszka edited the manuscript. All authors made critical revision before manuscript submission.
Acknowledgements: This work was supported by the Medical University of Silesia grant (KNW 1-087/N/5/0).
Conflict of interests: None declared.
REFERENCES
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46-54.
- Cintolo M, Costantino G, Pallio S, Fries W. Mucosal healing in inflammatory bowel disease: maintain or de-escalate therapy. World J Gastrointest Pathophysiol 2016; 7: 1-16.
- Sipponen T. Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil-derived biomarkers calprotectin and lactoferrin. Dig Dis 2013; 31: 336-344.
- Moniuszko A, Gluszek S, Rydzewska G. Rapid fecal calprotectin test for prediction of mucosal inflammation in ulcerative colitis and Crohn disease: a prospective cohort study. Pol Arch Intern Med 2017; 127: 312-3187.
- Hagel AF, De Rossi T, Konturek PC, et al. Plasma histamine and tumor necrosis factor-alpha levels in Crohn’s disease and ulcerative colitis at various stages of disease. J Physiol Pharmacol 2015; 66: 549-556.
- Szczeklik K, Krzysciak W, Domagala-Rodacka R, et al. Alterations in glutathione peroxidase and superoxide dismutase activities in plasma and saliva in relation to disease activity in patients with Crohn's disease. J Physiol Pharmacol 2016; 67: 709-715.
- Yan L, Borregard N, Kjeldsen N, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 by NGAL. J Biol Chem 2001; 276: 37258-37265.
- Chakraborty S, Kuar S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase-associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 2012; 1826: 129-169.
- Janas RM, Ochocinska A, Snitko R, et al. Neutrophil gelatinase-associated lipocalin in blood in children with inflammatory bowel disease. J Gastroenterol Hepatol 2014; 29: 1883-1889.
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 1996; 38: 414-420.
- Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase associated lipocalin is up-regulated in human epithelial cells by IL-1β, but not by TNF-α. J Immunol 2003; 171: 6630-6639.
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin is a novel early urinary biomarker for ischaemic renal injury. J Am Soc Nephrol 2003; 14: 2534-2543.
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231-1238.
- Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 2010; 120: 4065-4067.
- Martensson J, Bell M, Xu S, et al. Association of plasma neutrophil gelatinase-associated lipocalin (NGAL) with sepsis and acute kidney dysfunction. Biomarkers 2013; 18: 349-356.
- Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, Rydzewska G. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015; 44: 448-452.
- Budzynska A, Nowakowska-Dulawa E, Marek T, Boldys H, Nowak A, Hartleb M. Differentiation of pancreatobiliary cancer from benign biliary strictures using neutrophil gelatinase-associated lipocalin. J Physiol Pharmacol 2013; 64: 109-114.
- McLean MH, Thomson AJ, Murray GI, Fyfe N, Hold GL, El-Omar EM. Expression of neutrophil gelatinase-associated lipocalin in colorectal neoplastic progression: a marker of malignant potential? Br J Cancer 2013; 108: 2537-2541.
- Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004; 432: 917-921.
- Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2007; 18: 407-413.
- Manfredi MA, Zurakowski D, Rufo PA, Walker TR, Fox VL, Moses MA. Increase incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1091-1096.
- Nielsen OH, Gionchetti P, Ainsworth M, et al. Rectal dialysate and fecal concentration of neutrophil gelatinase-associated lipocalin, interleukin 8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol 1999; 94: 2923-2928.
- Oikonomou KA, Kapsoritakis AN, Theodoridou C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol 2012; 47: 519-530.
- Yesil A, Gonen C, Senates E, et al. Relationship between neutrophil gelatinase-associated lipocalin (NGAL) levels and inflammatory bowel disease type and activity. Dig Dis Sci 2013; 58: 2587-2593.
- Thorsvik S, Damas JK, Granlund AV, et al. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J Gastroenterol Hepatol 2017; 32: 128-135.
- de Bruyn M, Arijs I, Wollants WJ, et al. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2014; 20: 1198-1207.
- de Bruyn M, Arijs I, De Hertogh G, et al. Serum neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate marker for mucosal healing in patients with Crohn's Disease. J Crohns Colitis 2015; 12: 1079-1087.
- Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505-512.
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625-1629.
- Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439-444.
- Bolignano D, Della Torre A, Lacquaniti A, Costantino G, Fries W, Buemi M. Neutrophil gelatinase-associated lipocalin levels in patients with Crohn’s disease undergoing treatment with infliximab. J Invest Med 2010; 58: 569-571.
A c c e p t e d : December 15, 2017