PANCREATITIS-ASSOCIATED PROTEIN-1 SUPPRESSES APOPTOSIS
IN CERULEIN-STIMULATED PANCREATIC ACINAR CELLS IN RESPONSE
TO NUCLEAR FACTOR-KAPPA B ACTIVATION
INTRODUCTION
Pancreatitis is an inflammatory condition of the pancreas that can be reversible (acute pancreatitis) or irreversible (chronic pancreatitis) (1). Chronic pancreatitis results in fibrosis and often leads to pancreatic cancer. Acute pancreatitis accounts for the majority of the cases, most of which are mild and resolvable. However, a significant number of the cases become severe and ultimately fatal. The factors that control the severity of the disease are not well understood and, importantly, there are no therapeutic agents currently available for treatment.
There are several experimental models for acute pancreatitis. Rodents in which pancreatitis is induced by administration of an exogenous agent such as the peptide hormone cholecystokinin 8 (CCK) or cerulein, an analog of CCK, are used as animal models of the human disease (2-4). Cerulein treatment, which is most widely used in the laboratory to induce the mild acute pancreatitis phenotype, causes infiltration of inflammatory cells within the pancreas, pancreatic edema, and pancreatic acinar cell vacuolization (5). CCK or cerulein-stimulated pancreatic acinar cells are most commonly used for cellular studies of the biochemical pathways that underpin inflammation and cell death (6-9). Previous studies have shown that cerulein-treated pancreatic acinar cells experience oxidative stress, inflammation, and apoptosis (10, 11). The bile salt-induced acute pancreatitis model has been used as a model of severe acute pancreatitis, showing pancreatic necrosis (12, 13). Sodium taurocholate (0.2 to 0.3 mL of 3% to 5% sodium taurocholate) is infused into the duodenum in a retrograde fashion. Severe hemorrhagic necrosis of the pancreas is induced in a relatively short period. However, the damage to the pancreas is not uniform. Choline-deficient, ethionine-supplemented (CDE) diet-induced acute pancreatitis shows acute hemorrhagic pancreatitis with a mortality rate reaching nearly 100% (14). This model is somewhat limited to young female mice; 4- to 6-week-old female mice weighing 10 to 14 g. Due to the constant mortality rate, this model is used to evaluate the efficacy of new drugs for development to treat pancreatitis. Arginine-induced pancreatitis is a severe necrotizing pancreatitis. It is induced by a single intraperitoneal injection of high dose L-arginine (500 mg/100 g body weight) in rats (15). Pancreatic acinar cells regenerate and pancreatic architecture becomes almost normal after 14 days. However, the pathogenic mechanism is not yet fully understood. In pancreatic duct-ligation model, bile refluxes into the pancreatic duct induces acute pancreatitis (16). Even though the pancreatic duct has been obstructed by the tumor, the validity of this model is questionable. In the present study, we used cerulein pancreatitis in vitro model using AR42J cells since cerulein induces oxidative stress, inflammation, and apoptosis in pancreatic acinar cells, which is similarly shown in mild form of human acute pancreatitis.
PAP-1 (aka HIP, p23 or Reg2 protein) is a C-type lectin secreted in the pancreas during the acute phase of pancreatitis (17, 18) and is also known to be associated with inflammatory diseases including Crohn’s disease (19-21). In sodium taurocholate-induced severe necrotizing pancreatitis, inhibition of PAP-1 by siRNA or antisense oligodeoxyribonucleotides of PAP-1 worsened pancreatitis in rats (22, 23). Upregulation of PAP1 expression inhibited oxidative stress- or L-arginine-induced cell death and apoptosis of pancreatic acinar AR42J cells while down-regulation of PAP-1 increased cell death and apoptosis (24, 25). The studies indicate that PAP-1 have protective effect on cell death and apoptosis in several pancreatitis models. Although its exact biological function in the pancreas is unknown, it is generally believed that PAP-1 acts as an anti-inflammatory factor in several acute pancreatitis models and pancreatic acinar AR42J cells (26, 27). AR42J cells were widely used for the studies on acute pancreatitis as an in vitro model as shown by Jaworek et al. (28). They found that melatonin metabolite attenuates acute pancreatitis using cerulien-treated AR42J cells.
NF-κB is activated under the condition of oxidative stress (29), which is known to prevail in acute pancreatitis, and to result from excessive production of reactive oxygen species (ROS) (30). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is the major source of ROS during acute inflammation whereas NF-κB is the major target of ROS and redox signaling in acute pancreatitis (31). Cerulein-induced acute pancreatitis is known to trigger NF-κB activation (32) and, thus, our investigation began with testing the correlation between cerulein-induced up-regulation of PAP-1 gene expression and cerulein-induced activation of NF-κB using pancreatic acinar AR42 J cells as the experimental platform.
In the present study, to determine whether NADPH oxidase activity is required for cerulein-induced up-regulation of PAP-1 and NF-κB, two independent methods were used to suppress NADPH oxidase activity during induction. First, the small molecule DPI was used to inhibit activity of NADPH oxidase and second, the formation of the active, membrane associated enzyme complex was blocked by inhibiting translation of NADPH oxidase subunits p22phox and p47phox with mRNA-directed AS ODNs.
In addition, to test the potential relationship between cerulein-induced activation of NF-κB and up-regulation of PAP-1 gene transcription, activation of NF-κB was blocked by using a degradation-resistant mutant of the cytoplasmic NF-κB inhibitor IκBα (MAD3) to stably sequester cellular NF-κB in an inactive complex within the cytoplasm.
In the present study, we used cerulein pancreatitis in vitro model using AR42J cells since cerulein induces oxidative stress, inflammation, and apoptosis in pancreatic acinar cells, which is similarly shown in human acute pancreatitis. We have investigated PAP-1 function in the context of NF-κB-regulated apoptosis in cerulein-stimulated pancreatic acinar cells. Herein, we report that PAP-1 inhibits apoptosis in response to cerulein-induced NF-κB activation which is mediated with NADPH oxidase activation in pancreatic acinar AR42J cells.
MATERIALS AND METHODS
Cell culture
Rat pancreatic acinar AR42J cells (pancreatoma, ATCC CRL 1492) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (GIBCO-BRL, Grand Island, NY) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin).
Experimental protocol
AR42J cells (1 × 105/mL) treated with or without NADPH oxidase inhibitor diphenyleneiodonium (DPI, 10, 25, and 50 µM, Sigma-Aldrich, St. Louis, MO, USA) or transfected with antisense oligonucleotide (AS ODN) or sense oligonucleotide (S ODN) for NADPH oxidase subunits p22phox and p47phox, MAD3 double-point IkBa mutant (MAD3), or PAP-1 gene (PAP-1). The cells were incubated with cerulein (1 × 10–7 M, Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes (for the determination of NF-κB-DNA binding activity), 4 hours (for the determination of PAP-1 mRNA level), or 24 hours (for determination of viable cell numbers and DNA cleavage). For dose-response determinations, the cells were treated with various concentrations of cerulein and cultured for 4 hours prior to PAP-1 mRNA assay. For time-course measurements, the cells were treated with 1 × 10–7 M cerulein and cultured for 0.5 – 3 h prior to assay.
MAD3 and PAP-1 gene cloning and cell transfection
The mutated IκBα gene, known as MAD3 double-point mutant (substitution of serine residues at positions 32 and 36 by alanine residues) was prepared as described previously (33). The PAP-1 gene was prepared by PCR amplification of the full-length cDNA encoding the rat-specific PAP-1 gene using custom primers to generate KpnI and BamHI cleavage sites at the 5’- and 3’-ends, respectively. The sequences of the primers used are as follows: GACAGGATCCGAGGA GCAGAAAGATGATG and TGTCGGATCCTTTTAACCTGTAAATTTGCAGAC. The PCR product was digested with KpnI and BamHI and ligated with linearized pcDNA3 vector (Invitrogen Corp., Carlsbad, CA, USA). The pcDNA-PAP-1 clone was verified by DNA sequence analysis. Subconfluent AR42J cells were transfected for 16 hours with 10 µg of pcDNA3 (control), pcDNA-MAD3 or pcDNA-PAP-1 using N-[1-(2,3-dioleoyloxy) propyl]-N,N,N trimethylammonium methylsulfate (DOTAP, Boehringer-Mannheim).
Preparation of and cell transfection with AS ODN and S ODN for NADPH oxidase subunit p22phox and p47phox mRNAs
Custom phosphorothioate-modified oligonucleotides (ODNs) were obtained from GIBCO-BRL. The sequences of p22phox AS ODN and S ODN used are: GATCTGCCCC ATGGTGAGGACC and GGTCCTCACCATGGGGCAGATC, respectively (3).
The sequences of the p47phox AS ODN and S ODN used are: CTGTTGAAGTACTCGGTGAG and CTCACCGAGTACTTC AACAG, respectively (3).
The ODNs were pre-incubated with 0.5 µM DOTAP for 15 minutes before addition to the AR42J cells. The transfected cells were incubated for 24 hours before use.
Determination of cellular PAP-1 mRNA
PAP-1 mRNA was amplified using total cellular RNA, custom primers and the reverse transcription polymerase chain reaction (RT-PCR). PAP-1 primers: 5’- ACACCTTGTATCT GTGCTCAATGTAGC-3’ (forward primer) and 5’- CAAACT AAAGCTGTTTGCTGTCTGGTA -3’ (reverse primer) were used to provide the 337 bp PCR product. β-actin mRNA served as an internal control.
Electrophoretic mobility shift assay (EMSA) for NF-κB
Nuclear extracts were prepared as previously described (6). The NF-κB gel shift oligonucleotide (5’-ACTTGAGGGGACTTTCCCAGGGC-3’) was radiolabeled using [32P]-dATP (Amersham Biosciences, Piscataway, NJ, USA) and T4 polynucleotide kinase (GIBCO, Grand Island, NY, USA). The radiolabeled oligonucleotide was separated from unconsumed [32P]-dATP using a Bio-Rad purification column (Bio-Rad Laboratories) eluted with Tris-EDTA buffer. Nuclear extracts of the cells were incubated with the [32P]-labeled oligonucleotide in buffer containing 12% glycerol, 12 mM HEPES (pH 7.9), 1 mM EDTA, 1 mM DTT, 25 mM KCl, 5 mM MgCl2 and 0.04 µg/mL poly[d(I-C)] at room temperature (15 – 25°C) for 30 minutes. The samples were subjected to electrophoretic separation at 4°C on a nondenaturing, 5% acrylamide gel. The gel was dried at 80°C for 2 hours after which time it was exposed at –80°C to a radiography film using intensifying screens.
Cell viability assay
Cell viability was determined by using a hemocytometer and the trypan blue exclusion test.
Enzyme-linked immunosorbent assay for DNA fragmentation
DNA fragmentation was determined by measuring the amount of oligonucleosome-bound DNA in the cell lysates. The nucleosomes were quantified using a sandwich ELISA assay (Cell Death Detection ELISAplus kit; Boehringer Mannheim GmbH, Germany). The relative increase in nucleosomes in the cell lysate, determined at 405 nm, was expressed as an enrichment factor.
TUNEL assay for DNA fragmentation
DNA fragmentation associated with cell death was detected by using an in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) apoptosis detection kit (Roche Molecular Biochemicals, Indianapolis, IN, USA). For the TUNEL analysis, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at 4°C and subjected to permeabilization with 0.1% sodium citrate, containing 0.1% Triton X-100, for 20 min at room temperature. The fixed and permeabilized AR42J cells were incubated with the TUNEL reaction mixture for 60 min at 37°C. The nuclei were also stained with 4’, 6’-diamino-2-phenylindole (DAPI). Fluorescein-labeled DNA, indicating DNA fragmentation, was analyzed by using a laser-scanning confocal microscope (Leica TCS-NT, Heidelberg, Germany). The percentage of TUNEL labeling was expressed as the number of TUNEL-positive nuclei divided by the total number of nuclei stained with DAPI.
Statistical analysis
One-way ANOVA, followed by Newman-Keul’s post hoc tests, was used for statistical analysis. All data are reported as the mean ± S.E. of four independent experiments. A P-value of 0.05 or less was considered statistically significant.
RESULTS
Cerulein induces PAP-1 expression in AR42J cells
We investigated the effect of cerulein on PAP-1 gene expression by measuring the level of PAP-1 mRNA in AR42J cells treated for 4 h with 1 × 10–10 – 1 × 10–7 M cerulein. As shown in Fig. 1A, the untreated cells do not contain detectable levels of PAP-1 mRNA whereas in the cerulein-treated cells, PAP-1 mRNA is increased in a dose dependent-manner. Next, the PAP-1 mRNA level was measured as a function of the cell incubation period with 1 × 10–7 M cerulein. Fig. 1B shows that the expression of PAP-1 mRNA is significant at 2 h and continues to increase for up to 4 h (Fig. 1B).
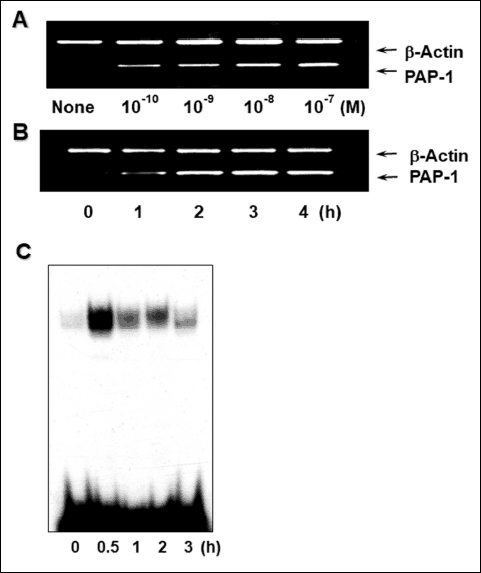 |
Fig. 1. Effects of cerulein concentration and incubation time on the levels of PAP-1 mRNA and active nuclear NF-κB in AR42J cells treated with cerulein. (A): Dose dependency of cerulein induced up-regulation of PAP-1 mRNA in AR42J cells. Cells were incubated without (‘None’) or with 1 × 10–10, 1 × 10–9, 1 × 10–8 or 1 × 10–7 M cerulein for 4 hours prior to PAP-1 mRNA amplification by RT-PCR. β-actin served as the internal control. (B): The time-dependency of cerulein induced up-regulation of PAP-1 mRNA in AR42J cells. Cells were incubated with 1 × 10–7 M cerulein for 1, 2, 3, or 4 hours prior to RT-PCR PAP-1 mRNA amplification. ‘None’ refers to untreated cells. b-actin served as the internal control. (C): DNA-binding activity of nuclear NF-kB determined by EMSA analysis for AR42J cells incubated with 1 × 10–7 M cerulein for 0.5, 1, 2 or 3 hours. The lane labeled ‘0’ corresponds to untreated cells. |
Cerulein induces NF-κB activation in AR42J cells
To determine the effect of cerulein on the level of active nuclear NF-κB, cells were treated with 1 × 10–7 M cerulein for up to 3 hours. During incubation time periods, cell samples were collected and the nuclear fractions were isolated for DNA-binding analysis. The results shown in Fig. 1C indicate that the amount of active nuclear NF-κB is significantly greater in cerulein-treated cells than in untreated cells, and that the greatest amount was observed for the 0.5-h treatment period.
Inhibition of NADPH oxidase by DPI and transfection with AS ODN suppresses cerulein-induced expression of PAP-1 and NF-κB activation in AR42J cells
In order to test the hypothesis that cerulein induces the expression of PAP-1 via NADPH oxidase-mediated activation of NF-κB, we first pretreated the cells with an exogenous inhibitor of NADPH oxidase catalytic activity, DPI, and measured the level of PAP-1 mRNA. For control experiments, PAP-1 mRNA levels in cells not treated with cerulein (‘None’ in Fig. 2A) and PAP-1 mRNA levels in cells treated with cerulein but not with DPI (‘Control’ in Fig. 2A) were measured. The increase in the level of PAP-1 mRNA induced by cerulein (‘Control’ versus ‘None’ in Fig. 2A) is prevented by the inhibition of NADPH oxidase catalytic activity with DPI (‘DPI’ in Fig. 2C).
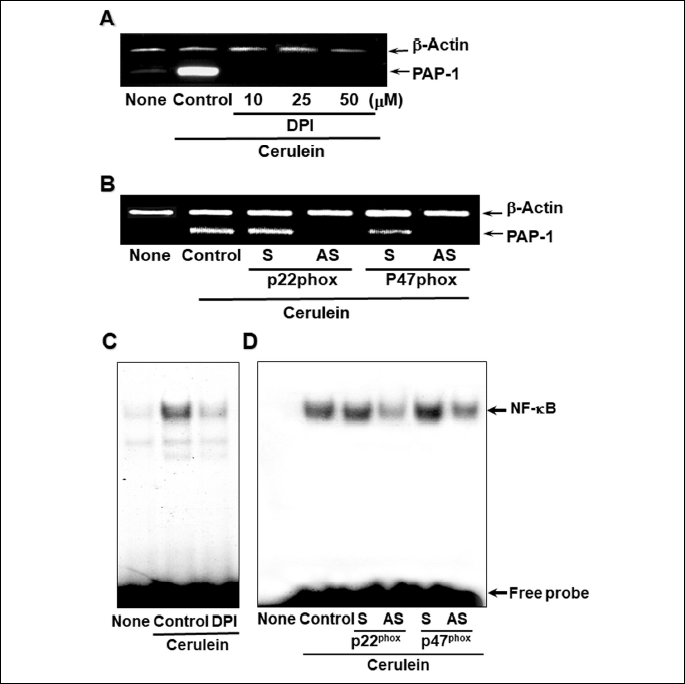
The involvement of NADPH oxidase in cerulein-induced expression of PAP-1 mRNA was also tested by inhibiting the expression of the cytoplasmic subunits (p22phox and p47phox) of NADPH oxidase and measuring the amount of PAP-1 mRNA in cerulein-treated AR42J cells. For this purpose, cells were transfected with phosphorothioate oligonucleotides (ODNs) that target and disrupt the translation of the NADPH oxidase subunits p22phox and p47phox. AR42J cells were transfected with the respective pairs of sense (‘S’ in Fig. 2B) and antisense (‘AS’ in Fig. 2B) ODNs and then treated with cerulein. The absence of cerulein-induced expression of PAP-1 mRNA in cells transfected with as AS ODN contrasts with the similar levels of PAP-1 observed for nontransfected cells, and for cells transfected with the corresponding S ODN. Taken together, these results show that blocking NADPH oxidase activity prevents the up-regulation of the PAP-1 gene.
The next step was to determine whether the reduction in NADPH oxidase activity by DPI, or by AS ODN directed at repression of p22phox and p47phox expression, also reduces the level of active nuclear NF-κB. The results show that the cerulein-induced increase in active nuclear NF-κB (‘Control’ versus ‘None’ in Fig. 2C) is blocked by DPI (‘DPI’ in Fig. 2C), and by AS ODN transfection (Fig. 2D).
Overexpression of IκBα double point mutant MAD3 suppresses cerulein-induced expression of PAP-1 and activation of NF-κB in AR42J cells
To determine if cerulein-induced PAP-1 gene expression is the result of NF-κB activation, we carried out an experiment in which NF-κB is blocked and determined whether PAP-1 gene expression is affected. For this purpose, we used the IκBα mutant MAD3, which is resistant to degradation via the ubiquitin-proteasome pathway. Overexpression of MAD3 is predicted to sequester the cellular NF-κB in an inactive complex and thus prevents its function as a transcriptional activator. Accordingly, AR42J cells were transfected with the IκBα mutant gene cloned in pcDNA (‘MAD3’ in Fig. 3), or for a control experiment, with the expression vector alone (‘pcDNA’ in Fig. 3). The cerulein-induced increase in the PAP-1 mRNA level was observed to be significantly diminished in the cells expressing the IκBα mutant gene (Fig. 3A). As anticipated, the level of active nuclear NF-κB is also reduced in cerulein-stimulated cells transfected with the IκBα mutant (Fig. 3B). These results suggest that NF-κB mediates the induction of PAP-1 gene expression in cerulein-stimulated cells.
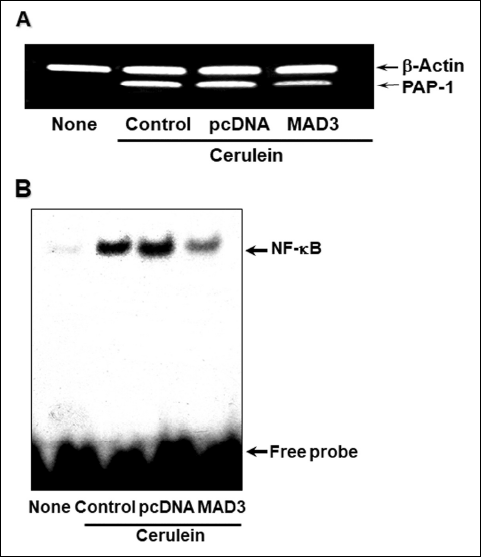 |
Fig. 3. The effect of overexpression of the IκBα mutant MAD3 on cerulein-induced up-regulation of PAP-1 gene expression and NF-κB activation. (A): AR42J cells were incubated with the pcDNA clone containing the IκBα mutant gene MAD3 (‘MAD3’) or the expression vector alone (‘pcDNA’), and with 1 × 10–7 M cerulein for 4 hours prior to PAP-1 mRNA amplification by RT-PCR. β-actin served as the internal control. ‘None’ refers to cells not having been transfected or exposed to cerulein, whereas ‘Control’ refers to cells treated with cerulein alone. (B): DNA-binding activity of nuclear NF-κB determined by EMSA analysis for AR42J cells incubated with MAD3 or pcDNA and 1 × 10–7M cerulein for 30 minutes. |
Overexpression of PAP-1 prevents cerulein-induced cell death and DNA fragmentation
To investigate whether PAP-1 protects AR42J cells from cerulein-induced cell death, the impact of overexpression of PAP-1 on the viability of cells transfected with the PAP-1 gene was examined by using the trypan blue exclusion test. As shown in Fig. 4A, the decrease in viable cell number in cell cultures treated with cerulein (‘None’ versus ‘Control’ in Fig. 4A) was largely reversed by transfection with the expression vector pcDNA containing the PAP-1 gene, but not by pcDNA alone (‘PAP-1’ and ‘pcDNA’ versus ‘Control’ in Fig. 4A).
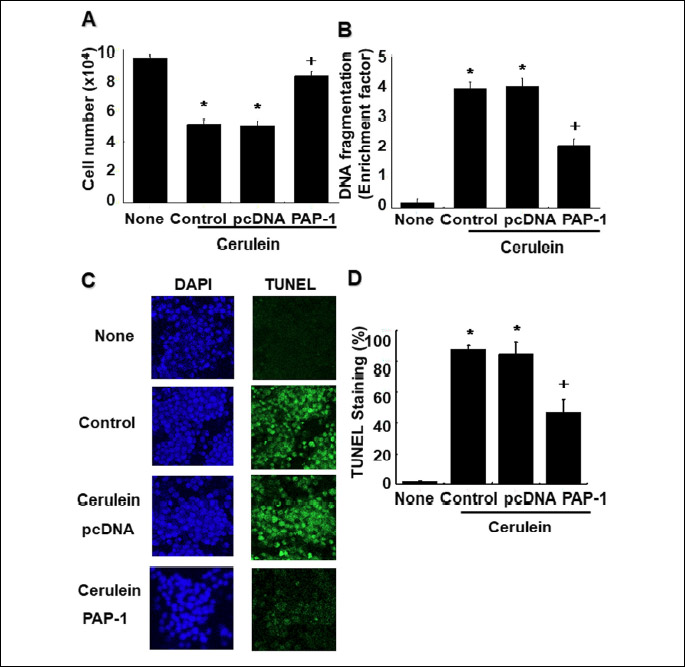
Next, we tested whether PAP-1 protects AR42J cells from cerulein-induced death through blocking the apoptotic pathway, by determining the impact of PAP-1 overexpression on DNA fragmentation. First, we employed the sandwich ELISA assay to quantitate oligonucleosomal DNA in cell lysates. As shown in Fig. 4B, the increase in DNA fragmentation resulting from cell treatment with cerulein (‘None’ versus ‘Control’) is significantly diminished in cells transfected with the pcDNA-PAP-1 clone but not by the pcDNA expression vector alone (‘PAP-1’ and ‘pcDNA’ versus ‘Control’ in Fig. 4B).
Lastly, to verify our findings, we used the TUNEL assay to detect double strand-cleaved DNA in the nuclei of whole cells. The images of cerulein-induced AR42J cells stained with DAPI (for total DNA) or with TUNEL (for cleaved DNA) are reported in Fig. 4C while the relative quantities of TUNEL-stained DNA associated with the imaged cells are reported in Fig. 4D. Comparison the images of DAPI-stained cells with TUNEL-stained cells reveals DNA fragmentation in cerulein-stimulated cells (‘Control’ in Fig. 4C) that is not observed for untreated cells (‘None’ in Fig. 4C). The reduction in cerulein-induced DNA fragmentation resulting from PAP-1 overexpression is evident from the comparison of the image of cells transfected with the PAP-1 expression vector with that of cells transfected with the expression vector alone. Fig. 4D shows that PAP-1 overexpression reduces DNA fragmentation by roughly 50%. Taken together, these findings indicate that PAP-1 functions to protect cells from cerulein-induced apoptosis.
DISCUSSION
During acute pancreatitis, the production of secreted digestive enzymes such as amylase and lipase is down-regulated and the synthesis of certain other proteins, one being PAP-1, is up-regulated (17, 18). Although the biological function of PAP-1 is unknown, the observation that PAP-1 expression has been found in the inflammatory tissues of pancreas, small intestine, and colon (19-21), prompted us to investigate the relationship of PAP-1 to key mediators of the inflammatory response. Recent study shows that NF-κB activation via facilitating its phosphorylation and nuclear translocation, accelerated inflammatory response and pancreatic cell injury in acute pancreatitis (34).
In this study, we focused our attention on the possible role of transcriptional regulator NF-κB in PAP-1 gene expression in pancreatic acinar cells. In the present study, the cells respond to cerulein exposure increased PAP-1 mRNA expression in a dose- and time-dependent manner, and increased the amount of active nuclear NF-κB in a timeframe in consistent with its posited role in transcriptional regulation of PAP-1. For the study on the role of NADPH oxidase activity on cerulein-induced up-regulation of PAP-1 and NF-κB, both DPI and AS ODNs for p22phox and p47phox greatly diminished the increase in PAP-1 mRNA and NF-κB activation which were induced in response to cerulein.
For the relationship between activation of NF-κB and up-regulation of PAP-1 in cerulein-stimulated cells, we here found that the transfection of the cells with the mutant of the cytoplasmic NF-κB inhibitor IκBα (MAD3) suppresses the increase in active nuclear NF-κB, and PAP-1 mRNA. The results provide strong evidence that cerulein-induced up-regulation of PAP-1 is mediated by NF-κB.
In the final phase of our investigation, we tested the potential role of PAP-1 in inhibition of cerulein-induced apoptosis by overexpressing PAP-1 in cells transfected with a PAP-1 gene-cloned expression vector. First, we observed that the decrease in cell viability resulting from exposure to cerulein is largely supressed in the transfected cells. Second, we found that DNA cleavage, which is the hallmark of cell apoptosis, is significantly increased in cerulein-induced cells, but much less so in cells in which PAP-1 is overexpressed. These findings suggest that at high levels, PAP-1 may function in an anti-apoptotic role. We postulate that PAP-1 expression may be one of the protective responses to cerulein-induced cell death. We note, however, that PAP-1 function may be context dependent. In particular, Qui et al. (35) recently reported that REG3A (another name for PAP-1) overexpression suppresses gastric cancer cell invasion and proliferation, and promotes apoptosis through the PI3K/Akt signaling pathway.
Lastly, we wish to point out that NF-κB-mediated regulation of PAP-1 expression has been observed in other systems. Specifically, Malka et al. (36) reported that pretreatment with NF-κB inhibitors, such as pyrrolidine dithiocarbamate, and the inhibitory peptide SN50, significantly reduced PAP-1 expression and apoptosis in TNF-α-stimulated pancreatic acinar cells. In addition, Cao et al. (37) presented evidence that Lactobacillus promotes the expression of REG3A in gut epithelial cells through receptor LRRC19-mediated NF-κB signaling pathways.
In the present study, the cells were incubated with cerulein for 4 hours for the determination of PAP-1 mRNA level. As shown in Fig. 1B, expression of PAP-1 mRNA increased for 4 hours. Therefore, we used 4 hour-time point for determination of PAP-1 mRNA expression for the following experiments on the effect of NADPH oxidase inhibition (using DPI or AS ODNS for p22phox and p47phox) and NF-κB inhibition (using mutant of NF-κB inhibitor IκBα) on cerulein-induced up-regulation of PAP-1 mRNA expression (Figs. 2 and 3).
PAP isoforms are identified in rats (PAP-1, PAP-2, PAP-3), mice (Reg3α, Reg3β, Reg3γ), humans (PAP, Reg3), canine (PAP), bovine (PAP), and sheep (PAP) (38). In multiple alignments of species and isoform using clustalW analysis, conservation of the structure for PAP proteins showed between 47 and 91% in species (38). Dexamethasone and IL-6 (Dex/IL6) induces all three PAP isoforms (PAP I, II and III) in AR42J cells (39). All three PAP genes (PAP I, II and III) are expressed in necrotizing pancreatitis model using bile salt sodium taurocholate (NaT) (23). In cerulein-induced acute pancreatitis, expression of PSP/reg and PAP I, II, and III are shown in exocrine pancreas (40).
In relation of PAP isoform and inflammatory cascades, PAP-1 has been shown to inhibit inflammation in macrophages and pancreatic acinar cells (26, 41). However, inactive PAP-2 mutant decreased inflammatory cytokines in macrophage (38). siRNA knockdown of PAP-3 in isolated sciatic nerves successfully suppressed its macrophage chemoattractant activity (42). The data indicate that three PAP isoforms are shown to have different immunologic functions. Therefore, it is necessary to assess the expression of PAP isoforms in cerulein-stimulated pancreatic acinar cells and experimental models of acute pancreatitis to determine role of PAP on cell death in pancreatic acinar cells associated with acute pancreatitis.
In experiments using AS ODN to inhibit RNA transcription and thereby the synthesis of the gene product, sense ODN (S ODN) and scrambled ODN have been usually used as controls. Many studies have used S ODN as control ODN (43-45). In the present study, we generated S ODN as a control for AS ODNS experiments. As shown in Fig. 2B, treatment of S ONN has no effect on expression of PAP-1 and activation of NF-κB compared to AS ODN treatment.
Regarding the overexpression of PAP-1 using PAP-1 gene transfection, we previously demonstrated that mRNA expression of PAP-1 was highly induced by treatment of PAP-1 gene in pancreatic acinar cells (24). In the present study, we obtained transfection efficiencies around 50 – 70%.
In conclusion, we have used cerulein-induced pancreatic acinar cells as an experimental platform to investigate the significance of PAP-1 up-regulation in acute pancreatitis and discovered that PAP-1 may function to prevent apoptosis in response to ROS-induced NF-κB signaling in pancreatic acinar cells.
Abbreviations: AS, antisense; DAPI, 4’,6’-diamino-2-phenylindole; DCF-DA, dichlorofluorescein diacetate; DPI, diphenyleneiodonium; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen; NF-κB, nuclear factor kappaB; ODN, oligonucleotides; PAP-1, pancreatitis-associated protein; REG3A, regenerating islet-derived 3α; ROS, reactive oxygen species; S, sense; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling
Authors’ contribution: H. Kim conceived of and designed the experiments; J.W. Lim assisted in experimental design; J.H. Yu performed the experiments; H. Kim and J. W. Lim analyzed the data; J. H. Yu wrote the paper; H. Kim reviewed and edited the paper. All authors agree with the edited version.
Acknowledgments: This study was supported in part by a Brain Korea 21 PLUS Project, College of Human Ecology, Yonsei University, Seoul, South Korea.
Conflict of interests: None declared.
REFERENCES
- Habtezion A, Gukovskaya AS, Pandol SJ. Acute pancreatitis: a multifaceted set of organelle and cellular interactions. Gastroenterology 2019; 146: 1941-1950.
- Tomaszewska R, Dembinski A, Warzecha Z, Banas M, Konturek SJ, Stachura J. Platelet activating factor (PAF) inhibitor (TCV-309) reduces caerulein- and PAF-induced pancreatitis. A morphologic and functional study in the rat. J Physiol Pharmacol 1993, 43: 345-352.
- Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-κB activation is associated with hormone-induced pancreatitis. Am J Physiol 1998; 275: G1402-G1414.
- Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endoc 2014; 47: 212-216.
- Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 2017; 8: 10-25.
- Yu JH, Lim JW, Kim H, Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol 2005; 37: 1458-1469.
- Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic Biol Med 2005; 39: 590-602.
- Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci 1989; 10: 418-423.
- Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation calcium mobilization and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol 1989; 257: G202-G209.
- Kim H. Cerulein pancreatitis: oxidative stress, inflammation, and apoptosis. Gut Liver 2008; 2: 74-80.
- Liu S, Zou H, Wang Y, et al. miR-155-5p is negatively associated with acute pancreatitis and inversely regulates pancreatic acinar cell progression by targeting Rela and Traf3. Cell Physiol Biochem 2018; 51: 1584-1599.
- Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol 1980; 15: 411-416.
- Aho HJ, Ahola RA, Tolvanen AM, Nevalainen TJ. Experimental pancreatitis in the rat. Changes in pulmonary phospholipids during sodium taurocholate-induced acute pancreatitis. Res Exp Med (Berl) 1983; 182: 79-84.
- Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol 1975; 79: 465-480.
- Tani S, Itoh H, Okabayashi Y, et al. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci 1990; 35: 367-374.
- Opie EL. The relation of cholelithiasis to disease of the pancreas and to fat-necrosis. Bull Johns Hopkins Hosp 1901; 12: 182-188.
- Closa D, Motoo Y, Iovanna JL. Pancreatitis-associated protein: from a lectin to an anti-inflammatory cytokine. World J Gastroenterol 2007; 13: 170-174.
- Keim V, Iovanna JL, Rohr G, Usadel KH, Dagorn JC. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology 1991; 100: 775-782.
- Masciotra L, Lechene de la Porte P, Frigerio JM, Dusetti NJ, Dagorn JC, Iovanna JL. Immunocytochemical localization of pancreatitis-associated protein in human small intestine. Dig Dis Sci 1995; 40: 519-524.
- Dieckgraefe BK, Stenson WF, Korzenik JR, Swanson PE, Harrington CA. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics 2000; 4: 1-11.
- Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet 2001; 10: 445-456.
- Lin YY, Viterbo D, Mueller CM, et al. Small-interference RNA gene knockdown of pancreatitis-associated proteins in rat acute pancreatitis. Pancreas 2008; 36: 402-410.
- Zhang H, Kandil E, Lin YY, Levi G, Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol 2004; 39: 870-881.
- Lim JW, Song JY, Seo JY, Kim H, Kim KH. Role of pancreatitis-associated protein 1 on oxidative stress-induced cell death of pancreatic acinar cells. Ann NY Acad Sci 2009; 1171: 545-548.
- Motoo Y, Taga K, Su SB, Xie MJ, Sawabu N. Arginine induces apoptosis and gene expression of pancreatitis-associated protein (PAP) in rat pancreatic acinar AR4-2J cells. Pancreas 2000; 20: 61-66.
- Vasseur S, Folch-Puy E, Hlouschek V, et al. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem 2004; 279: 7199-7207.
- Ortiz EM, Dusetti NJ, Vasseur S, et al. The pancreatitis-associated protein is induced by free radicals in AR4-2J cells and confers cell resistance to apoptosis. Gastroenterology 1998; 114: 808-816.
- Jaworek J, Szklarczyk J, Bonior J, et al. Melatonin metabolite, N1-acetyl-N1-formyl-5-methoxykynuramine (AFMK), attenuates acute pancreatitis in the rat: in vivo and in vitro studies. J Physiol Pharmacol 2016; 67: 411-421.
- Moldogazieva NT, Mokhosoev IM, Feldman NB, Lutsenko SV. ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic Res 2018; 52: 507-543.
- Criddle DN. Reactive oxygen species, Ca (2+) stores and acute pancreatitis; a step closer to therapy? Cell Calcium 2016; 60: 180-189.
- Jeong YK, Kim H. A mini-review on the effect of docosahexaenoic acid (DHA) on cerulein-induced and hypertriglyceridemic acute pancreatitis. Int J Mol Sci 2017; 18: E2239. doi: 10.3390/ijms18112239
- Steinle AU, Weidenbach H, Wagner M, et al. NF-κB/Rel activation in cerulein pancreatitis. Gastroenterology 1999; 116: 420-430.
- Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-κB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest 2004; 84: 49-62.
- Gu L, Ge Z, Wang Y, Shen M, Zhao P, Chen W. Double-stranded RNA-dependent kinase PKR activates NF-κB pathway in acute pancreatitis. Biochim Biophys Res Comm 2018; 503: 1563-1569.
- Qiu YS, Liao, GJ, Jiang, NN. REG3A overexpression suppresses gastric cancer cell invasion, proliferation and promotes apoptosis through PI3K/Akt signaling pathway. Int J Mol Sci 2018; 41: 3167-3174.
- Malka D, Vasseur S, Bodeker H, et al. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology 2000; 119: 816-828.
- Cao S, Su X, Zeng B, et al. The gut epithelial receptor LRRC19 promotes the recruitment of immune cells and gut inflammation. Cell Rep 2016; 14: 695-707.
- Viterbo D, Bluth MH, Mueller CM, Zenilman ME. Mutational characterization of pancreatitis-associated protein 2 domains involved in mediating cytokine secretion in macrophages and the NF-κB pathway. J Immunol 2008; 181: 1959-1968.
- Kandil E, Lin YY, Bluth MH, Zhang H, Levi G, Zenilman ME. Dexamethasone mediates protection against acute pancreatitis via upregulation of pancreatitis-associated proteins (PAP). World J Gastroenterol 2006; 12: 6806-6811.
- Graf R, Schiesser M, Lussi A, Went P, Scheele GA, Bimmler D. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, and PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J Surg Res 2002; 105: 136-144.
- Folch-Puy E, Granell S, Dagorn JC, Iovanna JL, Closa D. Pancreatitis-associated protein I suppresses NF-κB activation through a JAK/STAT-mediated mechanism in epithelial cells. J Immunol 2006; 176: 3774-3779.
- Namikawa K, Okamoto T, Suzuki A, Konishi H, Kiyama H. Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J Neurosci 2006; 26: 7460-7467.
- Ohno S, Yoshimoto M, Honda S, et al. The antisense approach in amyloid light chain amyloidosis: identification of monoclonal Ig and inhibition of its production by antisense oligonucleotides in in vitro and in vivo models. J Immunol 2002; 169: 4039-4045.
- Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol 2001; 36: 706-716.
- Pina-Medina AG, Hansberg-Pastor V, Gonzalez-Arenas A, Cerbon M, Camacho-Arroyo I. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids 2016; 105: 19-25.
A c c e p t e d : December 30, 2019