TRANSCRIPTIONAL COFACTOR DYXIN MEDIATES HYPERTROPHIC RESPONSE IN THE HEART DURING ANGIOTENSIN II-INDUCED HYPERTENSION
INTRODUCTION
The GATA family of transcription factors are important regulators of the cardiac hypertrophic response and heart failure (1). They interact with several co-factors including the LIM proteins, which are a large and a rather heterogenous group of proteins that have been proposed to be a part of the mechanosensitive apparatus in the actin cytoskeleton (2). The LIM proteins seem to have a role in cardiac mechanotransduction as well, since the upregulation of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) induced by the biomechanical stress has been shown to be attenuated in the cardiac myocytes deficient for the muscle LIM proteins (3). In addition, increased expression of a sarcomeric Z-disc LIM protein dyxin, also known as LIM and cysteine-rich domains 1 (LMCD1), has been described in response to mechanical stretch of the cardiac myocytes by us and others (4, 5) .
The dyxin protein consists of two carboxy-terminal LIM-domains and a cysteine-rich amino-terminal domain which all can participate in protein-protein interactions (6, 7). Dyxin has been shown to interact with GATA1/4/6-transcription factors and potentially affect their functions (8). For example, dyxin represses GATA6 activation of cardiac tissue-specific promoters (8). Previous studies have demonstrated that dyxin regulates both skeletal muscle and cardiac hypertrophy (4, 9, 10). The overexpression of dyxin leads to cardiomyocyte hypertrophy in neonatal rat cardiac myocytes whereas an inhibition of the dyxin gene blunted this response to hypertrophic stimuli (4). In addition, the cardiac-specific overexpression of dyxin in transgenic mice led to a significantly enhanced hypertrophic response (4), and augmented fibrosis after aortic banding (9) via an activation of calcineurin pathway.
Since dyxin has been proposed to have a key role in the development of cardiac hypertrophy, the aim of this study was to examine the effect of the adenoviral dyxin overexpression on cardiac function and gene expression in the normal heart and during angiotensin II (Ang II)-induced hypertension in rats.
MATERIALS AND METHODS
Experimental animals
Male 2-to-3-month-old Sprague-Dawley (SD) rats weighing 250–300 g from the colony of the Center of Experimental Animals at the University of Oulu, Finland, were used. All rats were kept in plastic cages with free access to tap water and regular rat chow in a room with a controlled 40% humidity and a temperature of 22°C. A 12 h light and 12 h dark environmental light cycle was maintained.
All experimental protocols were approved by the Animal Use and Care Committee of the University of Oulu and the Provincial Government of Southern Finland, Department of Social Affairs and Health, Finland. The study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Recombinant adenoviral vectors
The dyxin-overexpressing adenovirus was generated by subcloning the HindIII- EcoRV fragment from the rat dyxin cDNA into the Ad1-RSV shuttle vector (5). Putative adenovirus clones were plaque-purified, screened for inserts, propagated, isolated and titered to produce viral stocks with titers >2×109 pfu/mL. The recombinant replication-deficient adenovirus type 5 (Ad5) expressing nuclear localization signal (nls) -β-lactosidase (nls-lacZ) was generated as previously described (11, 12). The viral stocks with titers >2×109 pfu/mL were produced in Turku Centre for Biotechnology (BTK) virus core (Turku, Finland).
Experimental design
Male Sprague-Dawley rats were anesthetized with medetomidine hydrochloride (Domitor 250 µg/kg, intraperitoneally) and ketamine hydrochloride (Ketamine 50 mg/kg, i.p.). A left thoracotomy and a pericardial incision were made. Adenovirus-mediated gene transfer was performed as a local intramyocardial injection (1.5×109 in a 100-µL total volume) with a Hamilton precision syringe directly into the anterior wall of the left ventricle (LV) as previously described (13). After the operation, the anesthesia was partially antagonized with atipamezole hydrochloride (Antisedan 1.5 mg/kg, i.p.) and the rats were given buprenorphine hydrochloride (Temgesic, 0.05–0.2 mg/kg, subcutaneously) for analgesia. Angiotensin (Ang) II (Sigma Chemicals Corp. St Louis, MO, USA) (33 µg/kg/h) or vehicle (0.9% NaCl) was administered through subcutaneously implanted osmotic minipumps (Alzet, Durect Corporation, Cupertino, CA, USA) in conscious rats for 1 and 2 weeks (14).
Echocardiography
Transthoracic echocardiography was performed using a commercially available Acuson Ultrasound System (SequoiaTM 512) and a 15-MHz linear transducer (15L8) (Acuson, Mountain View, CA, USA) (15, 16). Rats were anesthetized with ketamine (50 mg/kg i.p.) and xylazine (10 mg/kg i.p.), and placed in the supine position, normal body temperature being maintained during the examination. Using two-dimensional imaging, a short axis view of the LV at the level of the papillary muscles was obtained, and a two dimensionally guided M-mode recording through the anterior and posterior walls of the LV was acquired. LV end-systolic dimensions (LVESD) and end-diastolic dimensions (LVEDD) as well as the thickness of the interventricular septum and posterior wall were measured from the M-mode tracings. LV fractional shortening (FS) and ejection fraction (EF) were calculated from the M-mode LV dimensions using the following equations: FS(%) = {(LVEDD-LVESD)/LVEDD}×100 and EF(%) = {(LVEDD)3-(LVESD)3/LVEDD3}×100.
For evaluation of LV diastolic function, mitral flow was recorded from an apical 4-chamber view. The peak flow velocities of the early rapid diastolic filling wave (E) and the late diastolic filling wave (A) were measured, and the E/A ratio was calculated. An average of three measurements of each variable was used. Echocardiographic measurements were performed by a skilled sonographer blinded to the treatments. After echocardiography, the animals were sacrificed, their hearts were weighed, and the tissue samples were immersed in liquid nitrogen and stored at –70°C for later analysis.
Isolation and analysis of RNA
The total RNA from the left ventricular tissues was isolated by the guanidine thiocyanate-CsCl method as described (5). RNA was analyzed by a real-time quantitative polymerase chain reaction (RT-PCR) with TaqMan chemistry on an ABI 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA) as previously described (5). The primer and probe sequences for the RNA detection are presented in Table 1. The results were normalized to 18S mRNA quantified from the same samples.

Histological analysis
For immunohistochemical analysis, the hearts were fixed in 10% buffered formalin solution. Transversal sections of the left ventricle were embedded in paraffin, and 5-µm-thick sections were cut. Sections were deparaffinized in xylene and dehydrated in graded ethanol, pretreated with Tris-EDTA buffer (pH 9), and to identify cellular localization of dyxin, incubated with a specific anti-dyxin polyclonal antibody prepared in rabbits (Davids Biotechnology, Regensburg, Germany) at the dilution of 1:2000. The primary antibody was detected by a peroxidase conjugated Dako REAL™ EnVision™ Detection System, Peroxidase/DAB kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions, and the samples were counterstained with hematoxylin.
Statistical analysis
For statistical analysis, data was first tested for normality by using the Kolmogrov-Smrinov and Shapiro-Wilk test. For normally distributed variables, unpaired Student’s t-test or one-way analysis of variance (ANOVA) followed by LSD (least significant difference) post hoc for multiple comparisons was performed. For data that was not normally distributed, statistical analysis was performed by using a non-parametric Welch t-test or Kruskal-Wallis one-way analysis of variance for multiple comparisons. A value of P<0.05 was considered statistically significant. Results are expressed as mean ±standard error of the mean (SEM).
RESULTS
Adenoviral gene delivery increases the left ventricular dyxin levels in vivo
To investigate the role of dyxin in the heart, the rats were subjected to in vivo intramyocardial gene transfer (13) to locally increase the dyxin levels in the rat myocardium. A significant increase of the dyxin gene expression was observed by quantitative RT-PCR following the injection of dyxin expressing adenoviral constructs into the LV free wall at 1.5×109 infectious units (Fig. 1). The efficiency of gene transfer protocol has been established in earlier study by X-gal staining of the Lac Z-injected hearts, which showed a large transmural staining area in the anterior wall of the left ventricle (16). In previous studies, we have also shown that adenoviral gene transfer is able to rescue failing myocardium after experimental myocardial infarction in rats in the studies with Gata4 (17) as well as in wild-type (WT) p38 kinase and constitutively active MKK3b experiments (13).
 |
Fig. 1. Cardiac-specific activation of dyxin gene expression by adenoviral gene delivery into the adult rat left ventricle. Dyxin mRNA levels were measured by quantitative RT-PCR from left ventricular tissue samples three days, one week and two weeks after dyxin gene transfer. Open bars represent LacZ and solid bars dyxin. The results are expressed as mean ± SEM (n = 5 in each group). **P<0.01, ***P<0.001 versus LacZ treated animals (Student’s test). |
The peak increase in dyxin mRNA levels was observed at day three after intramyocardial injections when compared to the LacZ-treated control animals, and dyxin mRNA levels remained significantly elevated throughout the two-week follow-up period. The localization of cardiac dyxin after the gene delivery was studied by the immunohistochemistry. In both groups, dyxin was localized in the endothelium of some larger vessels (weak staining, not shown) but in dyxin group there were also some vessels with strong endothelial reaction. In the LacZ-treated hearts, a weak signal was detected in the cytoplasm of some cardiac myocytes in the inflammatory area surrounded by the adenovirus injection site (Fig. 2). In the dyxin-treated hearts this cytoplasmic staining was often clearly stronger, and sometimes it was also localized in the nuclei of the myocytes.
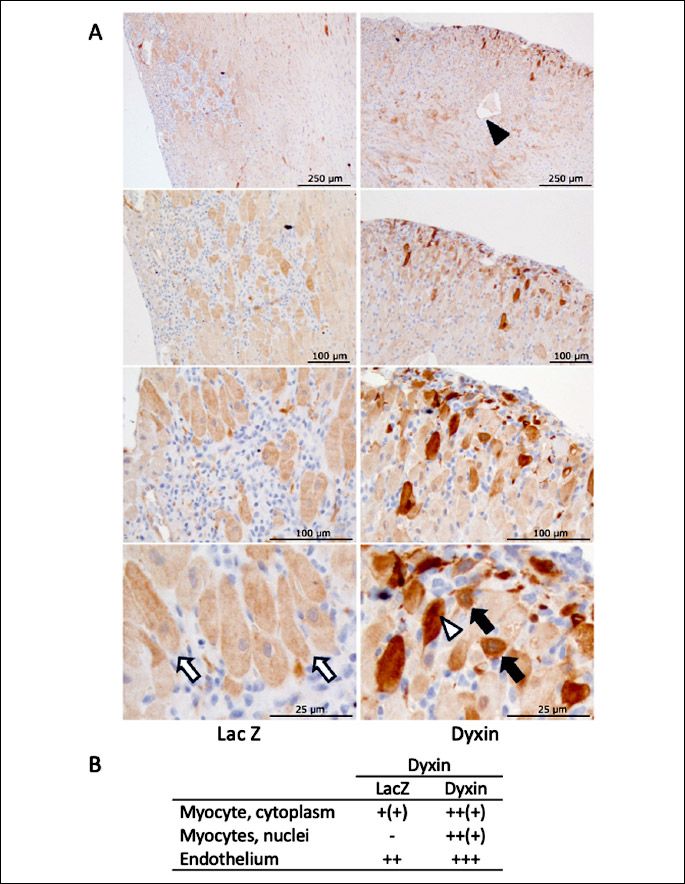
Dyxin regulates cardiac gene expression in vivo
Since dyxin is a transcriptional regulator protein which interacts with important regulators of a hypertrophic response such as transcription factors of GATA family, we analyzed changes in mRNA levels of selected genes of cardiac hypertrophic process (Fig. 3). The intramyocardial dyxin overexpression increased LV mRNA levels of a-myosin heavy chain (P<0.05), skeletal a-actin (P<0.05), and Na+/Ca2+-exchanger (NCX) (P<0.05) at one week (Fig. 3A, 3C and 3E) and cardiac α-actin (P<0.05) at one and two weeks (Fig. 3D) compared to the LacZ-treated control hearts. In addition, the LV dyxin gene delivery decreased the gene expression of interleukin-6 (IL-6) (P<0.01) at one week (Fig. 3F) and ANP at 2 weeks (P<0.01) (Fig. 3H) compared to the control hearts. On the other hand, dyxin overexpression did not alter the gene expression of beta myosin heavy chain (β-MHC) (Fig. 3B) and BNP (Fig. 3G) in the normal rat left ventricle.
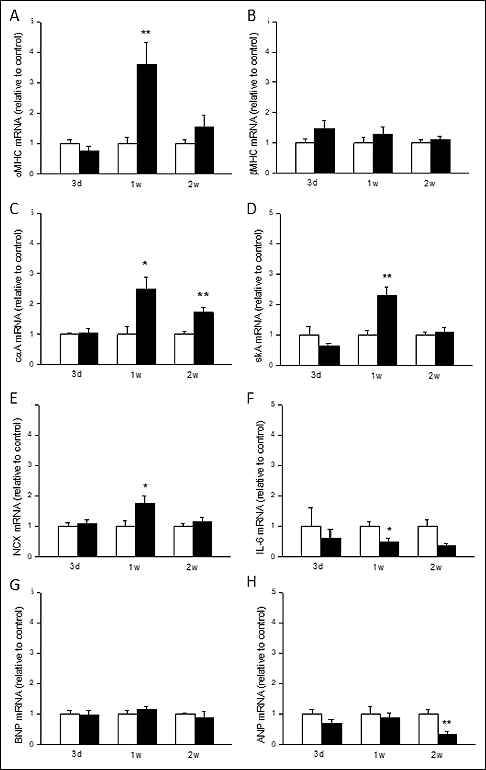 |
Fig. 3. The effect of intramyocardial dyxin gene transfer on mRNA levels of cardiac hypertrophy-associated genes in the normal adult rat heart. (A): α-myosin heavy chain (α-MHC); (B): β-MHC; (C): cardiac α-actin (cαA); (D): skeletal α-actin (skA); (E): Na+/Ca+ exchanger (NCX); (F): interleukin-6 (IL-6); (G): B-type natriuretic peptide (BNP) and (H): atrial natriuretic peptide (ANP) mRNA levels, measured by RT-PCR from the left ventricular tissue samples three days, one week and two weeks after the dyxin gene transfer. Open bars represent LacZ and solid bars dyxin. The results are expressed as mean ± SEM (n = 5 in each group). *P<0.05, **P<0.01 versus LacZ (Student’s test). |
The effect of the local intramyocardial dyxin gene delivery on cardiac structure and function in the normal rat heart
The cardiac function and structure were evaluated by echocardiography three days, one week and two weeks after in vivo intramyocardial adenoviral dyxin gene transfer. There were no statistically significant differences in the LV dimension (assessed as thickness of the posterior wall and interventricular septum, and diameter of left ventricle in both systole and diastole), LV ejection fraction (EF) and fractional shortening (FS) between the dyxin- and the LacZ-treated groups (Table 2). Based on the results of the echocardiography analysis, the overexpression of dyxin had no major effect on LV structure and function in the normal rat hearts during the two-week follow-up period.

The effects of adenoviral dyxin gene delivery in angiotensin II-induced hypertension in rats
Next, the effects of dyxin gene transfer on myocardial function and cardiac hypertrophy-associated gene expression were examined during Ang II-induced hypertension after one- and two-week systemic infusion of Ang II in adult rats (18). There was a trend towards altered LV dimensions by the dyxin gene transfer after one week (Table 3) and these differences were more pronounced at two weeks (Fig. 4, Table 4). The LV weight (LVW)/body weight (BW) ratio was increased both after one (LacZ: 2.1 ± 0.07, LacZ + Ang II: 2.5 ± 0.13, P<0.05; Dyxin: 2.0 ± 0.07, Dyxin + Ang II: 2.6 ± 0.12, P<0.01) and 2 weeks (LacZ: 2.1 ± 0.08, LacZ + Ang II: 2.5 ± 0.12, P<0.05; Dyxin: 2.2 ± 0.14, Dyxin + Ang II: 2.8 ± 0.09, P<0.01) of Ang II infusion compared to control rats, indicating Ang II induced cardiac hypertrophy. A significant increase in the posterior wall diameter in both systole (21%, P<0.05) and diastole (21%, P<0.001) as well as in the diameter of the interventricular septum in systole (19%, P<0.05) in the dyxin-treated group compared with the LacZ-treated group after two weeks of Ang II-infusion (Fig. 4) was observed. Although there was a trend of the adenoviral dyxin delivery to increase EF and FS at two weeks of Ang II infusion, these changes were not statistically significant (Table 4). Moreover, at one week after dyxin gene transfer, isovolumetric relaxation time was prolonged in the dyxin + Ang II group when compared to the dyxin group (Table 3). The Ang II infusion for two weeks prolonged the LV isovolumic relaxation time and the dyxin gene transfer non-significantly augmented it (Table 4).

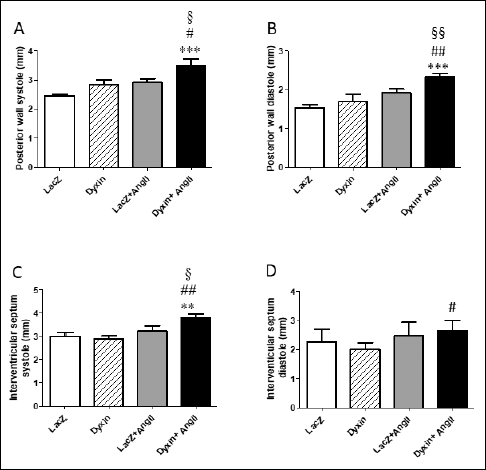 |
Fig. 4. The effect of the intramyocardial dyxin overexpression on cardiac structure during angiotensin II (Ang II)-induced hypertension in adult rats. (A-D): Structural parameters were analysed at two weeks by echocardiography in rats overexpressing dyxin and dose-matched LacZ control groups. The results are expressed as mean ± SEM (n = 4–8). **P<0.01, ***P<0.001 versus LacZ; #P<0.05 ##P<0.01 versus dyxin; §P<0.05, §§P<0.01 versus simultaneous dyxin gene transfer and Ang II infusion (One-way ANOVA followed by a least significant difference post hoc test. |

Finally, in view of changes in the gene expression in the normal rat heart at one week, we evaluated the effects of dyxin gene transfer at one week of Ang II infusion on cardiac hypertrophy-associated gene expression. As expected, Ang II infusion itself significantly increased ANP and BNP gene expression, whereas a significant decrease in the levels of ANP mRNA (55%, P<0.01) and BNP mRNA (68%, P<0.05) was observed (Fig. 5A and Fig. 5B) in the dyxin-treated group compared with the LacZ-treated group after one week of Ang II infusion. Moreover, the dyxin gene transfer caused a non-significant decrease in gene expression of collagens 1α1 and 3α1 (Fig. 5C and Fig. 5D).
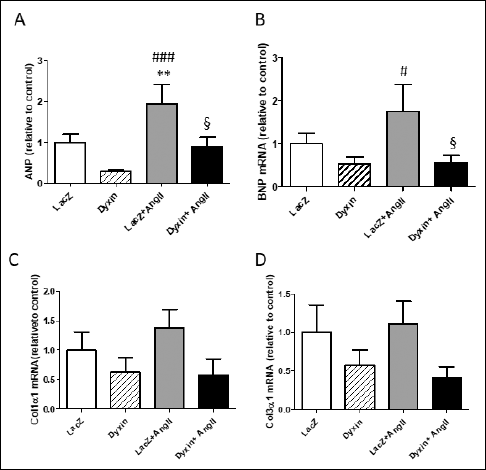 |
Fig. 5. Analysis of cardiac hypertrophy-associated gene expression in the left ventricle after the dyxin gene delivery during angiotensin II (Ang II)-induced hypertension. (A): Atrial natriuretic peptide (ANP); (B): B-type natriuretic peptide (BNP); (C): Collagen 1α1 (Col1α1) and (D): Collagen 3α1 (Col3α1) mRNA levels, measured by RT-PCR from left ventricular tissue samples one week after the dyxin gene transfer. The results are expressed as mean ± SEM, (n = 5–8). **P<0.01 versus LacZ; #P<0.01, ###P<0.001 versus dyxin; §P<0.05 versus simultaneous dyxin gene transfer and Ang II infusion (One-way ANOVA followed by least significant difference post hoc test.) |

DISCUSSION
Dyxin is a LIM domain protein previously shown to act in a co-transcription factor and upregulated in response to experimental cardiac stress (4, 5, 19). Notably, mechanical stretch induces dyxin gene expression in vitro as well as ex vivo (5). On the other hand, the overexpression of dyxin has resulted in cardiac myocyte hypertrophy together with an induction of hypertrophic gene program in vitro, and a knockdown of dyxin blunted these affects (4). Moreover, the overexpression of dyxin results in deterioration of cardiac hypertrophy during aortic banding in transgenic mice (9). According to these previous studies, dyxin may have a key role in the development of hypertensive heart disease. The aim of this study was to characterize the effect of the overexpression of dyxin on cardiac structure and function as well as on cardiac hypertrophy-associated gene expression during Ang II-induced hypertension.
The intramyocardial adenoviral mediated overexpression of dyxin resulted in a significant upregulation of cardiac dyxin gene expression at three days, and the dyxin mRNA levels remained significantly elevated throughout the entire follow-up period of two weeks. An immunohistochemical analysis showed that dyxin was localized in the cytoplasm of cardiac myocytes only in the inflammatory area caused by the intramyocardial injection in the LacZ-treated hearts, whereas in the dyxin-treated hearts, the immunoreactivity of dyxin was extensive and more pronounced compared to the LacZ-treated hearts. In addition to the cytoplasmic staining, dyxin was also localized in the nuclei of cardiac myocytes, and both the LacZ- and dyxin-treated hearts revealed the localization of dyxin also in the endothelium of larger vessels. Immunohistochemically assessed, no signs of myocardial failure such as fibrosis, myocyte hypertrophy or infiltration of immune cells were observed in the dyxin-overexpressed hearts. Altogether, these findings indicate that the adenoviral gene delivery increased dyxin levels in the cardiac myocytes, including the nuclei, but it did not induce any functional or structural changes in the healthy adult rat heart.
Previous studies have consistently showed the role of dyxin in the development of cardiac hypertrophy (4, 5, 9). In the present study, the overexpression of dyxin led to significant thickening of the posterior LV wall in both systole and diastole and the interventricular septum in systole during Ang II-induced cardiac hypertrophy, indicating that dyxin has a pivotal role in mediating the hypertrophic response during Ang II-induced pressure overload.
The Ang II administration by osmotic minipumps in conscious rats increases mean arterial pressure and causes cardiac hypertrophy and diastolic dysfunction (as reflected by a decreased E/A ratio and a prolonged LV isovolumic relaxation time) but has no major effect on LV systolic function (fractional shortening or ejection fraction) (18, 20). In the present study, the overexpression of dyxin in left ventricles resulted in the upregulation of cardiac genes aMHC, Skα-A, NCX and Caα-A together with the decrease of IL-6 and ANP mRNA levels compared to the LacZ-treated rat hearts at one week. Interestingly, during Ang II-induced hypertension, an induction of genes of the cardiac hypertrophy program was blunted by the overexpression of dyxin compared to the LacZ-treated control hearts. Dyxin gene transfer tended to decrease mRNA levels of Col1α1 and Col3α1, although the overexpression of dyxin has been previously shown to aggravate cardiac fibrosis (9). Furthermore, LV ANP and BNP gene expressions were significantly downregulated by the overexpression of dyxin in Ang II-induced hypertension.
ANP and BNP are released into to the circulation during the cardiac remodeling process (21). Moreover, their plasma levels are higher in patients with heart failure compared to healthy people and decrease by the treatment with Ang II receptor blockers (ARB) or angiotensin converting enzyme (ACE) inhibitors while LVEF increases (22). In the present study, the adenoviral dyxin gene delivery into the left ventricle of rat hearts did not significantly alter systolic (a non-significant increase in LVEF and FS) or diastolic (a non-significant prolongation of LV isovolumic relaxation time) function during Ang II administration. Consequently, because the cardiac hormonal system is activated by the increased wall stretch due to pressure overload as well as during myocardial hypertrophy (23), the downregulation of LV ANP and BNP expressions due to the overexpression of dyxin might be related to the direct cardiac effects of dyxin. Of note, concentrations of BNP exhibit nonlinear associations with LV systolic function in healthy subjects, and both low and high concentrations have been shown to associate with reduced LV systolic function (24).
Altogether, the findings of this study indicate that during Ang II-induced hypertension dyxin acts in a synergistic manner together with Ang II mediating the hypertrophic response in the myocardium, and it seems to act as a distinct regulator of the cardiac hypertrophy-associated gene program. The knockdown of the cardiac dyxin may well be performed during Ang II-induced hypertension to demonstrate whether dyxin is a distinct regulator of the hypertrophic response in the myocardium. In addition, the studies conducted in normotensive and hypertensive animals at different ages would be needed to assess the effects of age-related changes in renin-angiotensin system (25). Since the nuclear envelope detects the contractile forces of the cytoskeleton during mechanical load and rearranges the chromatin resulting in changes in the cardiac gene expression and, subsequently, the activation of hypertrophic response (26), dyxin may also be a part of mechanosensitive machinery in the myocardium. Thus, our present and the previous findings (4, 5, 9) suggest that dyxin may be an important mediator of the hypertrophic response in hypertensive heart disease. However, in the model of Ang II induced hypertension, we cannot rule out the direct effects of Ang II per se. In the future, it would be interesting to investigate the mechanisms of the distinct effect of dyxin on cardiac hypertrophy-associated gene expression as well as the relation between dyxin and GATA4, another key transcriptional regulator of the hypertrophic gene program. One of the mechanisms to be studied could be inhibition of calcineurin pathway e.g. by salvianolate (Sal) (27) to determine the effect of this pathway in dyxin-mediated hypertrophic response (9). Moreover, whether inhibiting cardiac dyxin could be a therapeutic option for hypertensive heart disease, and if dyxin has a functional role also in ischemic heart disease, remain to be established.
Funding: This work was supported by grants from the Academy of Finland (JR, grant number 276747; HR, grant number 2666621), Aarne Koskelo Foundation, Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research and the Sigfrid Juselius Foundation.
Acknowledgements: We thank Ketlin Adel, Marja Arbelius, Pirjo Korpi, Kati Lampinen, Kaisa Penttila and Sirpa Rutanen for their expert technical assistance.
Conflict of interests: None declared.
REFERENCES
- Brewer A, Pizzey J. GATA factors in vertebrate heart development and disease. Expert Rev Mol Med 2006; 8: 1-20.
- Smith MA, Hoffman LM, Beckerle MC. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol 2014; 24: 575-583.
- Raskin AM, Hoshijima M, Swanson E, McCulloch AD, Omens JH. Hypertrophic gene expression induced by chronic stretch of excised mouse heart muscle. Mol Cell Biomech 2009; 6: 145-159.
- Frank D, Frauen R, Hanselmann C, et al. Lmcd1/Dyxin, a novel Z-disc associated LIM protein, mediates cardiac hypertrophy in vitro and in vivo. J Mol Cell Cardiol 2010; 49: 673-682.
- Luosujarvi H, Aro J, Tokola H, et al. A novel p38 MAPK target dyxin is rapidly induced by mechanical load in the heart. Blood Press 2010; 19: 54-63.
- Bespalova IN, Burmeister M. Identification of a novel LIM domain gene, LMCD1, and chromosomal localization in human and mouse. Genomics 2000; 63: 69-74.
- Wang J, Deng CY, Xiong YZ, et al. cDNA cloning, sequence analysis of the porcine LIM and cysteine-rich domain 1 gene. Acta Biochim Biophys Sin 2005; 37: 843-850.
- Rath N, Wang Z, Lu MM, Morrisey EE. LMCD1/Dyxin is a novel transcriptional cofactor that restricts GATA6 function by inhibiting DNA binding. Mol Cell Biol 2005; 25: 8864-8873.
- Bian ZY, Huang H, Jiang H, et al. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension 2010; 55: 257-263.
- Ferreira DMS, Cheng AJ, Agudelo LZ, et al. LIM and cysteine-rich domains 1 (LMCD1) regulates skeletal muscle hypertrophy, calcium handling, and force. Skelet Muscle 2019; 9: 26. doi: 10.1186/s13395-019-0214-1
- Charron F, Tsimiklis G, Arcand M, et al. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev 2001; 15: 2702-2719.
- Grepin C, Dagnino L, Robitaille L, Haberstroh L, Antakly T, Nemer M. A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol Cell Biol 1994; 14: 3115-3129.
- Tenhunen O, Rysa J, Ilves M, Soini Y, Ruskoaho H, Leskinen H. Identification of cell cycle regulatory and inflammatory genes as predominant targets of p38 mitogen-activated protein kinase in the heart. Circ Res 2006; 99: 485-493.
- Rysa J, Aro J, Ruskoaho H. Early left ventricular gene expression profile in response to increase in blood pressure. Blood Press 2006; 15: 375-383.
- Rysa J, Leskinen H, Ilves M, Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 2005; 45: 927-933.
- Tenhunen O, Soini Y, Ilves M, et al. p38 Kinase rescues failing myocardium after myocardial infarction: evidence for angiogenic and anti-apoptotic mechanisms. FASEB J 2006; 20: 1907-1909.
- Rysa J, Tenhunen O, Serpi R, et al. GATA-4 is an angiogenic survival factor of the infarcted heart. Circ Heart Fail 2010; 3: 440-450.
- Suo M, Hautala N, Foldes G, et al. Posttranscriptional control of BNP gene expression in Angiotensin II-induced hypertension. Hypertension 2002; 39: 803-808.
- Li A, Ponten F, dos Remedios CG. The interactome of LIM domain proteins: The contributions of LIM domain proteins to heart failure and heart development. Proteomics 2012; 12: 203-225.
- Serpi R, Tolonen AM, Huusko J, et al. Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats. Cardiovasc Res 2011; 89: 204-213.
- Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017; 14: 30-38.
- Menezes Falcao L, Pinto F, Ravara L, van Zwieten PA. BNP and ANP as diagnostic and predictive markers in heart failure with left ventricular systolic dysfunction. J Renin Angiotensin Aldosterone Syst 2004; 5: 121-129.
- Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev 2003; 24: 341-356.
- Lyngbakken MN, Kvisvik B, Aagaard EN, et al. B-type natriuretic peptide is associated with indices of left ventricular dysfunction in healthy subjects from the general population: The Akershus Cardiac Examination 1950 Study. Clin Chem 2021; 67: 204-215.
- Pasanen L, Launonen H, Siltari A, et al. Age-related changes in the local intestinal renin-angiotensin system in normotensive and spontaneously hypertensive rats J Physiol Pharmacol 2019; 70: 199-208.
- Ross JA, Stroud MJ. The NUCLEUS: Mechanosensing in cardiac disease. Int J Biochem Cell Biol 2021; 137: 106035. doi: 10.1016/j.biocel.2021.106035
- Chen C, Yu H, Zhu P, et al. The effect of salvianolate on cardiomyocyte remodeling improvement after myocardial infarction through calcineurin/nuclear factor C3 of the activated T cell/B-myosin heavy chain pathway regulation. J Physiol Pharmacol 2022; 73: 347-361.
A c c e p t e d : December 31, 2023